Speak directly to the analyst to clarify any post sales queries you may have.
A concise authoritative overview framing how antimicrobial stewardship, regulatory evolution, and supply dynamics are reshaping veterinary antibiotics strategy across sectors
Veterinary antibiotics occupy a pivotal position at the intersection of animal health, food security, and public health. Over recent years, pressure from antimicrobial resistance, regulatory reforms, and changing consumer expectations has pushed the sector into a period of accelerated transformation. Practitioners, producers, and policymakers now confront a more complex decision environment where therapeutic efficacy must be balanced against stewardship obligations and supply continuity.Against this backdrop, a rigorous synthesis of market dynamics, segmentation behaviors, and regulatory influences is essential for stakeholders to formulate resilient strategies. This executive summary consolidates cross-cutting trends, segmentation insights, regional considerations, competitive behaviors, and pragmatic recommendations to guide stakeholders operating across companion animal and livestock markets. The analysis emphasizes actionable clarity, linking observable shifts to operational levers that can be deployed immediately to manage risk and capture opportunities in a rapidly evolving landscape.
How antimicrobial stewardship, regulatory tightening, supply chain resilience and technology adoption are collectively transforming veterinary antibiotic use and procurement
The landscape for veterinary antibiotics is being reshaped by several transformative shifts that are simultaneously regulatory, technological, and market-driven. Foremost is the global intensification of antimicrobial stewardship programs, which are reducing routine prophylactic use and catalyzing more diagnostic-driven therapeutic decisions. This change is driving demand for rapid diagnostics and targeted treatment protocols, thereby altering procurement patterns and prescribing behaviors among veterinarians and producers.In parallel, regulatory tightening around residue limits, drug approvals, and import controls is elevating compliance costs while incentivizing innovation in formulations and delivery mechanisms. Supply chain fragility exposed by recent global disruptions has prompted stakeholders to diversify sourcing, evaluate vertical integration, and prioritize suppliers with robust quality assurance and local manufacturing capabilities. Moreover, the rise of alternative modalities-such as vaccines, probiotics, and non-antibiotic therapeutics-introduces competitive dynamics that are eroding some traditional volumes but opening channels for differentiated product portfolios.
Technological adoption is another major vector of change. Digital herd health platforms, precision dosing systems, and telemedicine for companion animals are influencing utilization patterns and enabling more granular stewardship reporting. Finally, shifting consumer attitudes toward antibiotic use in animal-derived food products are pressuring retailers and integrators to require improved traceability and documented reductions in antibiotic exposure, which in turn affects supplier selection and product development priorities. Together, these forces are accelerating a transition toward a more transparent, regulated, and innovation-oriented veterinary antibiotics ecosystem.
Assessing the multifaceted consequences of new United States tariff measures introduced in 2025 on supply chains, pricing dynamics and strategic sourcing across veterinary antibiotics
The imposition of new tariff measures by the United States in 2025 introduces an added layer of complexity into an already dynamic veterinary antibiotics landscape. Tariff-driven cost increases for imported active pharmaceutical ingredients and finished formulations can alter sourcing strategies, pushing manufacturers and distributors to reassess supplier footprints and inventory buffers. As a result, organizations may accelerate localization of critical inputs or pursue contractual hedges to mitigate margin compression and ensure uninterrupted availability for both companion animals and livestock.Beyond cost implications, tariffs can influence competitive positioning. Domestic producers with integrated supply chains may gain relative advantage in pricing and lead times, while multinational suppliers reliant on established export routes could face margin pressure that constrains reinvestment in R&D or pipeline expansions. Regulatory compliance and customs complexity associated with tariff regimes also create administrative burdens that can delay time-to-market for newer therapeutic agents, especially those requiring cross-border manufacturing steps.
From a strategic perspective, firms will need to evaluate the tariff impact across procurement, pricing, and contractual frameworks. Firms serving large-scale animal production customers are likely to face heightened negotiating pressure as buyers seek predictable input costs, while veterinary clinics and retail channels may confront retail price adjustments or shifts in product assortment. In response, stakeholders should consider scenario planning that integrates tariff pathways, supply reconfiguration options, and strategic inventory management to preserve therapeutic availability and marginal stability in the face of trade policy volatility.
Comprehensive segmentation intelligence illuminating class-level drug portfolios, animal type behaviors, end-user purchasing patterns and therapeutic area treatment imperatives
Segmentation insights reveal differentiated drivers and adoption patterns that vary by therapeutic class, animal type, end-user, and clinical indication. Analysis by class shows a wide spectrum of therapeutic categories, encompassing Aminoglycosides such as Amikacin, Apramycin, Gentamicin, Kanamycin, and Neomycin; Amphenicols represented by Azidamfenicol, Chloramphenicol, Florfenicol, Metiamycin, and Thiamphenicol; Cephalosporins including Cefalexin, Cefalonium, Cefazolin, Cefovecin, Ceftiofur, and Ceftriaxone; Fluoroquinolones such as Ciprofloxacin, Danofloxacin, Difloxacin, Enrofloxacin, Norfloxacin, and Orbifloxacin; Macrolides covering Azithromycin, Clarithromycin, Erythromycin, Gamithromycin, Spiramycin, Tilmicosin, Tulathromycin, and Tylosin; Other Quinolones including Cinoxacin, Nalidixic Acid, Oxolinic Acid, Pipemidic Acid, and Rosoxacin; Penicillins with Amoxicillin, Ampicillin, Cloxacillin, Oxacillin, Penicillin G, and Penicillin V; Polymixins such as Colistin/Polymyxin E and Polymyxin B; Sulfonamides including Sulfadiazine, Sulfamerazine, Sulfamethizole, Sulfamethoxazole, and Sulfasalazine; Tetracyclines comprising Chlortetracycline, Doxycycline, Oxytetracycline, and Tetracycline; and Trimethoprim combinations such as Trimethoprim Plus Sulfamethazine, Trimethoprim-Sulfadiazine, Trimethoprim-Sulfadimidine, Trimethoprim-Sulfadoxine, and Trimethoprim-Sulfamethoxazole.When viewed through the lens of animal type, distinct demand profiles emerge between companion animals and livestock. Companion animal care, driven by cats and dogs, emphasizes formulations and dosing regimens optimized for owner administration, palatability, and safety. By contrast, livestock categories including cattle, poultry, sheep and goats, and swine prioritize cost-effectiveness, group administration modalities, and regulatory compliance for residues. End-user segmentation highlights divergent purchasing patterns: animal production facilities focus on volume and long-term supplier relationships, household pet owners are driven by convenience and veterinarian recommendations, and veterinary clinics prioritize performance, diagnostic-backed prescribing, and product breadth.
Therapeutic area segmentation further clarifies use cases and innovation opportunities. Dermatological infections and soft tissue and wound care often require topical and combination therapies that balance immediate efficacy with stewardship considerations. Respiratory, gastrointestinal, systemic, reproductive system, and urinary tract infections each present different diagnostic and dosing complexities that influence delivery systems, formulation development, and stewardship protocols. Integrating insights across these multiple segmentation axes enables more precise targeting of R&D investments, commercial strategies, and stewardship interventions tailored to the needs and constraints of each sub-market.
Regional intelligence mapping how Americas, Europe Middle East & Africa and Asia-Pacific differ in regulation, supply chains, stewardship adoption and manufacturing dynamics
Regional dynamics exert a material influence on regulatory frameworks, supply chains, and adoption of stewardship practices. In the Americas, regulatory agencies and industry stakeholders are advancing stewardship programs while major production systems demand reliable therapeutic options compatible with rigorous residue monitoring and export requirements. Market participants in this region are balancing rapid adoption of diagnostics with efforts to secure resilient sourcing amid changing trade policies and tariff measures.Europe, Middle East & Africa exhibits heterogeneity driven by stringent European regulatory standards, emerging market growth in parts of the Middle East and Africa, and divergent infrastructure capacity. European markets are characterized by aggressive antimicrobial stewardship, strict approval pathways, and a strong preference for traceability and low-residue products, which drives demand for alternative therapeutics and advanced diagnostics. Markets in parts of the Middle East and Africa may demonstrate faster volume growth but require differentiated approaches to distribution, cold-chain integrity, and localized regulatory support.
Asia-Pacific continues to be a high-impact region due to its extensive livestock production systems, evolving regulatory regimes, and concentrated manufacturing capabilities. Here, stewardship initiatives are progressing unevenly, and large-scale producers are increasingly adopting management practices and technologies that reduce reliance on routine antibiotic use. The region’s manufacturing strengths also position it as a strategic source for global supply, prompting multinational firms and local players to navigate complex trade, quality assurance, and regulatory alignment challenges. Across these regions, stakeholders must tailor strategies to local regulatory pressures, supply chain realities, and customer expectations.
Competitive positioning and corporate strategies revealing how product innovation, partnerships, supply resilience and targeted R&D are redefining market leadership dynamics
Competitive dynamics in the veterinary antibiotics ecosystem reflect a mix of established pharmaceutical manufacturers, specialized veterinary biotech firms, generic producers, and contract manufacturers. Leading organizations are differentiating through investments in formulation innovation, diagnostics integration, and enhanced supply chain transparency. In particular, firms that pair antibiotic portfolios with diagnostic solutions or stewardship support programs are better positioned to retain market access amid tightening regulatory scrutiny.Operational excellence and supply chain continuity are central themes. Companies that have invested in diversified sourcing, local manufacturing capabilities, or strategic partnerships demonstrate increased resilience against trade and tariff shocks. Meanwhile, growth-oriented players are pursuing collaborative models such as strategic alliances with diagnostics providers, veterinary service networks, and feed and nutrition companies to create integrated value propositions that align with producer and clinician needs.
R&D strategies are also evolving. Rather than relying solely on incremental modifications to legacy molecules, many organizations are exploring novel delivery systems, targeted-release formulations, and adjunctive therapies that can reduce total antibiotic exposure while maintaining therapeutic outcomes. Finally, M&A and licensing activities continue to be tools for capability augmentation, enabling rapid entry into specialized therapeutic niches or geographic markets where regulatory knowledge and distribution channels confer competitive advantage.
Practical strategic recommendations for commercial leaders to fortify stewardship, supply resilience, product innovation and regional go-to-market execution
Industry leaders should prioritize a set of pragmatic actions to navigate regulatory pressure, trade volatility, and evolving clinical practice. First, integrate antimicrobial stewardship into core commercial propositions by coupling products with diagnostics, dosing guidance, and outcome monitoring support. Doing so will improve prescriber confidence, satisfy regulatory expectations, and create differentiation that is less vulnerable to price-based competition.Second, conduct a structured supply chain resilience assessment that identifies critical suppliers, single-source risks, and tariff exposure across production stages. Transition strategies may include qualified dual sourcing, targeted localization of critical inputs, and strategic inventory management to smooth short-term disruptions while maintaining cost discipline. Third, accelerate formulation and delivery innovation that prioritizes targeted therapies and reduced systemic exposure; this approach can help preserve therapeutic utility while aligning with residue and stewardship constraints.
Fourth, tailor go-to-market approaches by region and end-user. For livestock customers, emphasize cost-effective group administration options and demonstrable residue compliance; for companion animal channels, highlight palatability, safety, and convenience. Fifth, invest in stakeholder engagement with regulators and industry groups to shape feasible stewardship pathways and to prepare for evolving policy scenarios. Finally, embed scenario planning that models tariff outcomes, regulatory shifts, and emergent competitor moves so leadership teams can respond quickly and with validated contingency plans.
Transparent and rigorous mixed-methods research approach integrating practitioner interviews, regulatory review and multi-source triangulation to validate insights
The research methodology underpinning this analysis combined primary qualitative engagements, systematic regulatory review, and triangulated secondary evidence to ensure robust conclusions. Primary inputs included structured interviews with practicing veterinarians across companion animal and livestock practices, procurement and quality assurance professionals within production systems, and senior executives from pharmaceutical and diagnostics companies. These conversations provided real-world insights into prescribing behavior, supply constraints, and product selection drivers.Secondary research encompassed regulatory documentation, published clinical guidance on antimicrobial stewardship, patent landscape analysis, and public filings related to manufacturing and trade policy. Data from trade and customs reporting, along with industry whitepapers and clinical studies, were synthesized to map supply chains and identify potential tariff-sensitive nodes. Findings were triangulated through cross-validation between primary interviews and documentary evidence, and hypotheses were stress-tested in expert panel reviews to ensure interpretive validity.
Quality controls included standardized interview protocols, source auditing for regulatory citations, and methodological transparency around inclusion criteria for clinical and commercial evidence. The combined methodological approach is designed to yield actionable intelligence that reflects both practitioner realities and macro-level trends while minimizing bias through cross-source verification and expert adjudication.
Synthesis and strategic outlook emphasizing how stewardship, supply resilience and targeted innovation will determine long-term competitiveness in veterinary antibiotics
In sum, the veterinary antibiotics arena is undergoing a consequential realignment driven by antimicrobial stewardship imperatives, tighter regulation, trade policy shifts, and evolving therapeutic alternatives. Stakeholders who proactively adapt-by pairing therapeutic solutions with diagnostics, strengthening supply chains, and pursuing targeted innovation-will be better placed to sustain market access and maintain clinical efficacy while satisfying regulatory and societal expectations.Looking forward, the most successful organizations will treat stewardship and resilience as strategic assets rather than compliance burdens. By aligning commercial models with diagnostic-enabled prescribing, fostering regional manufacturing and sourcing agility, and prioritizing formulation approaches that reduce total antibiotic exposure, companies can both protect public health interests and preserve long-term commercial viability. The recommendations and insights provided here offer a pragmatic roadmap for decision-makers seeking to translate these macro trends into concrete operational and strategic moves.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Veterinary Antibiotics Market
17. Pakistan Veterinary Antibiotics Market
Companies Mentioned
- Ashish Life Science Private Limited
- Bimeda Inc.
- Biogénesis Bagó S.A.
- C.H. Boehringer Sohn AG & Co. KG
- Ceva Santé Animale
- Dechra Pharmaceuticals PLC
- Eco Animal Health Group PLC
- Elanco Animal Health Incorporated
- Intas Pharmaceuticals Ltd.
- Krka, d. d., Novo mesto
- Kyoritsuseiyaku Corporation
- LABORATORIO AVI-MEX
- LABORATORIOS CALIER, S.A.
- Merck KGaA
- Neogen Corporation
- Ourofino Group
- Sequent Scientific Ltd.
- Vetoquinol SA
- Virbac SA
- Zoetis Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
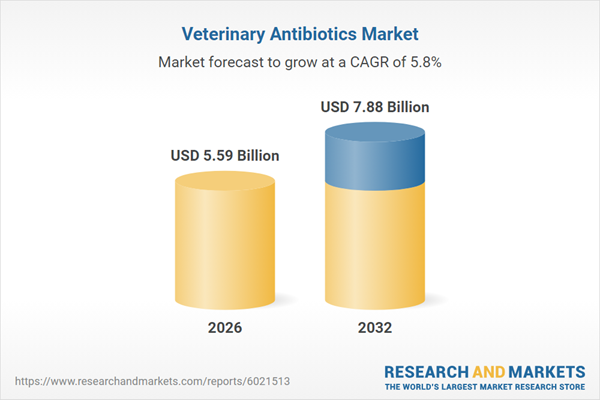
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 5.59 Billion |
| Forecasted Market Value ( USD | $ 7.88 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |