Speak directly to the analyst to clarify any post sales queries you may have.
A strategic framing of clinical priorities, device innovation, and delivery channel shifts that are redefining vitreoretinal surgical practice and procurement dynamics
This executive summary sets the stage by synthesizing clinical, technological, and commercial vectors reshaping vitreoretinal surgery. Surgeons are increasingly prioritizing patient comfort, shorter recovery, and enhanced visualization, while device developers respond with innovations in instrument miniaturization, integrated imaging, and lighting. At the same time, providers are reconfiguring care delivery through ambulatory surgery centers and high-volume outpatient networks, which has direct implications for device selection, procurement cycles, and service models.
Across clinical practice, procedural trends and the evolution of perioperative protocols have elevated the importance of ergonomics, single-use infection control, and compatibility with heads-up surgical displays. Consequently, innovation is not limited to hardware alone; it extends into software-driven imaging, interoperability with intraoperative OCT, and system-level workflow integration. These dynamics create converging pressures on manufacturers to deliver differentiated performance, streamlined sterilization logistics, and demonstrable improvements in surgeon efficiency and patient outcomes.
Strategic stakeholders-including device manufacturers, hospital procurement teams, and clinical leaders-must balance clinical utility with operational realities. This summary frames the critical considerations that influence purchasing decisions, product development roadmaps, and competitive positioning, and it highlights where targeted investments can yield outsized clinical and commercial returns.
How miniaturization, integrated imaging, and the rise of outpatient surgical venues are rapidly reshaping device design, purchasing behavior, and competitive value propositions
The landscape of vitreoretinal surgery is experiencing transformative shifts driven by technological convergence, care-site migration, and clinician preferences. Miniaturization of instrumentation and the widespread adoption of smaller gauge probes have altered procedural workflows, lowering surgical trauma and expanding eligibility for outpatient procedures. Simultaneously, advanced visualization systems, including three-dimensional displays and intraoperative OCT, are elevating intraoperative decision-making and enabling more predictable outcomes.
Additionally, the emergence of high-speed cutters, refined illumination technologies, and enhanced fluidics systems is improving tissue control and reducing operative times. These device-level advances are complemented by an increased emphasis on single-use disposables for infection control and operational simplicity, which in turn influences supply chain design and per-procedure economics. The move toward plug-and-play interoperability across visualization, imaging, and vitrectomy platforms supports integrated surgical ecosystems, creating opportunities for strategic partnerships and platform-led competition.
Operationally, the rise of ambulatory surgery centers and outpatient ophthalmic clinics changes device utilization patterns and drives demand for compact, easy-to-maintain systems. In short, the confluence of minimally invasive techniques, digital imaging, and care-site migration is reshaping vendor value propositions and compelling incumbents and new entrants alike to prioritize usability, integration, and lifecycle support.
Practical consequences and strategic responses to tariff-driven input cost volatility that require supply chain realignment, pricing innovation, and production localization initiatives
The introduction or escalation of United States tariffs in 2025 creates multifaceted implications for manufacturers, distributors, and healthcare providers engaged in vitrectomy device supply and procurement. Increased tariff barriers can raise landed costs for components or finished systems sourced from affected jurisdictions, prompting procurement teams to reassess vendor selection criteria and total cost of ownership. In response, some manufacturers may accelerate nearshoring or diversify supplier networks to mitigate exposure and secure critical components such as micro-cutters, optics, and electronic modules.
Moreover, tariffs can reshape pricing strategies and contractual negotiations with hospital systems and ambulatory surgery centers. Providers focused on predictable operating budgets may push for longer-term supply agreements or demand greater transparency in cost pass-through, while device firms may explore value-based service bundles or flexible leasing models to preserve market access. At the same time, tariffs can incentivize investment in domestic manufacturing capacity, which can be a strategic differentiator for companies seeking supply chain resilience, faster lead times, and stronger alignment with procurement policies that favor local production.
From an operational standpoint, distributors and aftermarket service teams will likely need to adapt logistics planning, inventory buffering, and reverse logistics to account for tariff-driven cost differentials. Overall, tariffs act as a catalyst for supply chain reconfiguration, pricing innovation, and closer collaboration between commercial teams and procurement stakeholders to preserve access and maintain clinical continuity.
In-depth segmentation insights revealing how device categories, gauge preferences, end-user settings, clinical applications, and material choices uniquely influence product selection and procurement
Segmentation-driven insight reveals differentiated demand patterns and clinical priorities across device categories, gauge sizes, end users, applications, and material strategies. When considering device category distinctions, clinicians and purchasing committees evaluate light sources for spectral quality and heat management, visualization systems for image fidelity and heads-up ergonomics, vitrectomy probes for cut rate and flow dynamics, and full vitrectomy systems for interoperability and servicing. Within visualization systems, the relative advantages of three-dimensional visualization and intraoperative OCT are weighed against capital intensity and training needs. For vitrectomy probes, the comparative trade-offs among 20G, 23G, 25G, and 27G instruments hinge on tissue handling, incision size, and surgeon familiarity.
Gauge size is a critical technical axis: 20G instruments retain utility in select complex cases where instrument rigidity and flow are priorities, whereas 23G, 25G, and 27G options facilitate minimally invasive approaches that support faster recovery and outpatient management. End users exhibit distinct procurement behaviors: ambulatory surgery centers favor compact, easy-to-maintain systems and disposables that streamline turnover, hospitals prioritize robust service contracts and multi-use platforms that support diverse case mixes, and ophthalmology clinics seek cost-effective, outpatient-suited instruments that align with procedural volumes and staffing models.
Applications such as diabetic retinopathy, macular hole, retinal detachment, and vitreous hemorrhage impose differing demands on instrumentation and visualization; for example, complex retinal detachments may necessitate higher-flow probes and advanced lighting, while macular surgery benefits most from superior magnification and intraoperative OCT guidance. Lastly, material strategies-disposable versus reusable-present trade-offs between infection control, per-procedure cost, logistics, and environmental considerations, and these choices are increasingly shaped by institutional infection protocols and sustainability commitments.
How distinct regional procurement patterns, regulatory environments, and care-delivery models across the Americas, Europe Middle East & Africa, and Asia-Pacific shape device adoption and commercial strategy
Regional dynamics drive distinct adoption rhythms, regulatory considerations, and supply chain strategies across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, clinical networks and the proliferation of ambulatory surgery centers shape demand for compact, integrated vitrectomy systems and disposables that support high-throughput outpatient workflows. Procurement decisions in this region are often influenced by hospital group purchasing organizations, care pathway standardization, and an emphasis on total cost of ownership and service reliability.
In Europe, the Middle East & Africa, regulatory diversity and fragmented procurement environments encourage flexible product configurations and responsive aftermarket support. Providers in these regions may place higher value on localization of technical service, adaptable financing options, and modular systems that can be tailored to varying levels of surgical infrastructure. Asia-Pacific presents a heterogeneous landscape where rapid capacity expansion in tier-one urban centers coexists with developing surgical markets, driving demand for scalable and cost-effective platforms. Local manufacturing capabilities, regulatory harmonization efforts, and strategic partnerships with regional distributors are particularly salient in Asia-Pacific.
Across all regions, considerations such as reimbursement environments, surgeon training infrastructure, and the availability of skilled technicians influence the pace of technology adoption. Cross-border supply chain planning, regulatory strategy, and tailored commercial models are therefore essential to maximize market access and long-term adoption across these geographic clusters.
Competitive positioning and strategic maneuvering among established platform providers and agile innovators that determine adoption through integration, service, and clinical evidence
Competitive dynamics in the vitrectomy device landscape show incumbents emphasizing platform integration, service networks, and clinician training, while innovators focus on disruptive imaging, single-use instrument technologies, and ergonomic visualization. Leading manufacturers maintain advantages through comprehensive portfolios that combine illumination, high-fidelity visualization, and vitrectomy mechanics, complemented by global service footprints and structured training programs for surgeons and operating room teams. This breadth supports long-term partnerships with large hospital systems and ambulatory centers that prioritize reliability and full lifecycle support.
At the same time, specialized companies and agile newcomers are making targeted investments in heads-up 3D visualization, intraoperative OCT integration, and ultra-high-speed cutting technologies. These focused innovations often lower the barrier to adoption for centers seeking clinical differentiation or improved throughput. Additionally, several firms are optimizing reusable-versus-disposable product lines to address infection-control priorities while balancing cost and environmental impact. Strategic collaborations between instrumentation manufacturers and imaging or software companies are also emerging as a route to create bundled clinical workflows that enhance surgeon decision-making and procedural efficiency.
From a commercial standpoint, successful companies combine robust clinical evidence generation, proactive engagement with key opinion leaders, and flexible commercial models that reflect diverse end-user requirements. In short, competitive advantage increasingly depends on the ability to deliver integrated solutions, rapid technical support, and demonstrable improvements in clinical and operational outcomes.
Actionable, high-impact steps executives should take to align product development, supply chain resilience, and commercial models with evolving clinical and procurement imperatives
Industry leaders seeking to strengthen their position should adopt a pragmatic, multi-pronged strategy that addresses product, supply chain, and commercial execution. First, invest in interoperability and modularity by ensuring visualization systems, illumination sources, and vitrectomy consoles work seamlessly together and integrate intraoperative imaging where clinically valuable. Second, prioritize supply chain resilience through supplier diversification, nearshoring options for critical components, and contingency inventory strategies that reduce exposure to tariff and logistics disruptions.
Third, tailor commercial models to the needs of different end users by offering flexible financing, leasing, and hemodynamic service bundles that align with ambulatory surgery center economics as well as hospital procurement preferences. Fourth, develop clear clinical value narratives supported by outcome-focused evidence and surgeon-led training programs to accelerate adoption, emphasizing operational benefits such as reduced procedural time and improved ergonomics. Fifth, balance disposable and reusable product portfolios by evaluating infection control imperatives, lifecycle costs, and sustainability goals, and by offering end-of-life services that minimize environmental impact.
Finally, engage proactively with payers and procurement groups to demonstrate value beyond list price, and invest in targeted partnerships with regional distributors and clinical educators to streamline market entry. Together, these actions will help companies convert technological advantage into sustained commercial traction.
A rigorous, clinician-centered research approach combining primary interviews, technical evaluation, and supply chain mapping to produce actionable device and commercial insights
The research methodology blends qualitative clinician insight with device-level technical assessment and supply chain analysis to produce a holistic view of the vitrectomy ecosystem. Primary research included structured interviews with vitreoretinal surgeons, operating room managers, procurement directors, and medical device engineers to capture frontline perspectives on usability, durability, and clinical priorities. These conversations provided nuanced input on instrumentation trade-offs, visualization preferences, and the clinical contexts that drive gauge selection and procedural technique.
Technical assessments evaluated device ergonomics, cutter mechanics, illumination characteristics, and imaging interoperability through bench testing and peer-reviewed clinical literature. Supply chain mapping examined supplier concentration, critical component sourcing, and service network density to identify potential points of vulnerability and resilience. Regulatory and reimbursement landscapes were analyzed comparatively to understand approval pathways, post-market surveillance expectations, and procurement constraints across major regions.
Triangulation of findings across these approaches enabled the generation of actionable insights without relying solely on singular data sources. Throughout, methodological rigor was maintained by validating manufacturer claims against clinical feedback and by cross-checking technical observations with independent surgical experience to ensure relevance and reliability for decision-makers.
A concise synthesis of technological, operational, and commercial forces that together determine which manufacturers and products will excel in modern vitreoretinal care delivery
In conclusion, the vitrectomy device landscape is characterized by rapid technological innovation, shifting care delivery models, and evolving procurement dynamics that together create both opportunities and operational challenges. Advances in miniaturization, high-fidelity visualization, and integrated intraoperative imaging are elevating procedural precision and expanding the clinical utility of minimally invasive approaches. Concurrently, the growth of ambulatory surgical venues and changing procurement preferences exert pressure on manufacturers to deliver compact, serviceable, and cost-transparent solutions.
Operational risks such as tariff-driven input cost volatility, supplier concentration, and logistics disruptions require proactive mitigation through diversified sourcing and localized capacity where feasible. Commercial success will increasingly hinge on the ability to demonstrate clinical value, offer flexible commercial arrangements, and provide robust training and service networks that reduce the friction of adoption. Ultimately, stakeholders that align technological innovation with practical operational and economic realities will be best positioned to support clinicians, improve patient outcomes, and secure durable commercial relationships in this evolving field.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
21. China Vitrectomy Devices Market
Companies Mentioned
The key companies profiled in this Vitrectomy Devices market report include:- Alcon Inc.
- Bausch + Lomb Corporation
- Blink Medical Ltd.
- BVI Medical, Inc.
- Carl Zeiss Meditec AG
- D.O.R.C. Holding B.V.
- Geuder AG
- Haag-Streit Holding AG
- HOYA Medical Singapore Pte. Ltd.
- Iridex Corporation
- Johnson & Johnson Vision Care, Inc.
- MedOne Surgical, Inc.
- Medtronic plc
- NIDEK Co., Ltd.
- OCULUS Optikgeräte GmbH
- Oertli Instrumente AG
- Peregrine Surgical LLC
- Synergetics USA, Inc.
- Topcon Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
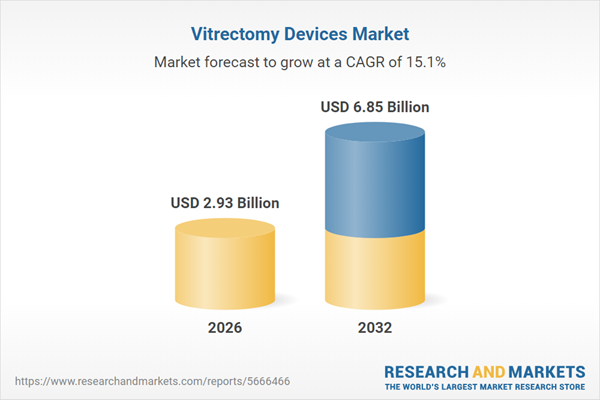
| Estimated Market Value ( USD | $ 2.93 Billion |
| Forecasted Market Value ( USD | $ 6.85 Billion |
| Compound Annual Growth Rate | 15.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |