Global Autoimmune Disease Therapeutics Market - Key Trends and Drivers Summarized
Why Are Autoimmune Disease Therapeutics Crucial in Modern Medicine?
Autoimmune disease therapeutics have become increasingly crucial in modern medicine due to the rising prevalence of autoimmune disorders worldwide. Autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, lupus, and inflammatory bowel disease, occur when the body's immune system mistakenly attacks its own tissues. These conditions can lead to chronic pain, disability, and significant morbidity, impacting the quality of life for millions of individuals. The growing understanding of the underlying mechanisms of these diseases has led to the development of targeted therapies that aim to modulate the immune system and reduce inflammation. Biologic drugs, which include monoclonal antibodies and fusion proteins, have revolutionized the treatment landscape by offering more effective and specific treatment options compared to traditional immunosuppressive therapies. The advancement in autoimmune disease therapeutics represents a significant leap in addressing these complex conditions and improving patient outcomes.How Are Technological Innovations Transforming Autoimmune Disease Treatment?
Technological innovations are transforming the treatment of autoimmune diseases, bringing new hope to patients and healthcare providers alike. One of the most significant advancements is the development of biologics, which are engineered to target specific components of the immune system. These therapies, such as TNF inhibitors and IL-6 blockers, have shown remarkable efficacy in managing symptoms and halting disease progression. Additionally, advancements in genomics and personalized medicine are enabling the identification of genetic markers associated with autoimmune diseases, allowing for more precise and tailored treatment approaches. Innovations in drug delivery systems, including sustained-release formulations and injectable devices, are enhancing the convenience and adherence to treatment regimens. Furthermore, the integration of digital health technologies, such as mobile health apps and wearable devices, is facilitating better disease monitoring and management. These technological strides are not only improving the effectiveness of autoimmune disease therapeutics but are also enhancing the overall patient experience.What Are the Key Challenges and Trends in Autoimmune Disease Therapeutics?
Despite the progress in autoimmune disease therapeutics, several challenges and trends shape the current landscape. One of the primary challenges is the high cost of biologic therapies, which can limit access for many patients. The development of biosimilars, which are lower-cost alternatives to biologics, is a growing trend aimed at addressing this issue and increasing accessibility. Another challenge is the management of side effects associated with long-term immunosuppressive therapy, which can lead to increased susceptibility to infections and other complications. Research is ongoing to develop safer and more tolerable treatment options. Additionally, the trend towards combination therapies, which use multiple drugs to target different pathways of the immune response, is gaining traction as a strategy to enhance treatment efficacy and minimize resistance. The increasing focus on early diagnosis and intervention is also a critical trend, as it can lead to better disease management and improved long-term outcomes. These challenges and trends highlight the dynamic nature of the autoimmune disease therapeutics field and the continuous efforts to improve patient care.What Factors Are Driving the Growth in the Autoimmune Disease Therapeutics Market?
The growth in the autoimmune disease therapeutics market is driven by several factors. Technological advancements in biologics and personalized medicine have significantly enhanced the efficacy and specificity of treatments, leading to better patient outcomes. The increasing prevalence of autoimmune diseases, driven by factors such as genetic predisposition, environmental triggers, and lifestyle changes, is expanding the patient population requiring treatment. Additionally, the growing awareness and improved diagnostic capabilities are enabling earlier detection and intervention, which is crucial for effective disease management. The development of biosimilars is also contributing to market growth by providing more affordable treatment options and increasing access to therapy. Regulatory support and favorable reimbursement policies are further encouraging the adoption of advanced therapeutics. Moreover, the continuous investment in research and development by pharmaceutical companies and strategic collaborations among key industry players are accelerating the innovation and availability of new treatments. These factors collectively contribute to the robust growth of the autoimmune disease therapeutics market, ensuring continued advancements in the management and treatment of these challenging conditions.Report Scope
The report analyzes the Autoimmune Disease Therapeutics market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Drug Class (Anti-inflammatory, Interferons, NSAIDs, Antihyperglycemics, Other Drug Class); Indication (Rheumatic Disease, Multiple Sclerosis, Inflammatory Bowel Disease, Type 1 Diabetes, Other Indications).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Autoimmune Disease Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Autoimmune Disease Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Autoimmune Disease Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Inc., AbbVie, Inc., Amgen, Inc., AstraZeneca PLC, Bristol-Myers Squibb Company and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 14 companies featured in this Autoimmune Disease Therapeutics market report include:
- Abbott Laboratories, Inc.
- AbbVie, Inc.
- Amgen, Inc.
- AstraZeneca PLC
- Bristol-Myers Squibb Company
- F. Hoffmann-La Roche AG
- Johnson & Johnson
- Novartis International AG
- Pfizer, Inc.
- UCB SA
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories, Inc.
- AbbVie, Inc.
- Amgen, Inc.
- AstraZeneca PLC
- Bristol-Myers Squibb Company
- F. Hoffmann-La Roche AG
- Johnson & Johnson
- Novartis International AG
- Pfizer, Inc.
- UCB SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 263 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
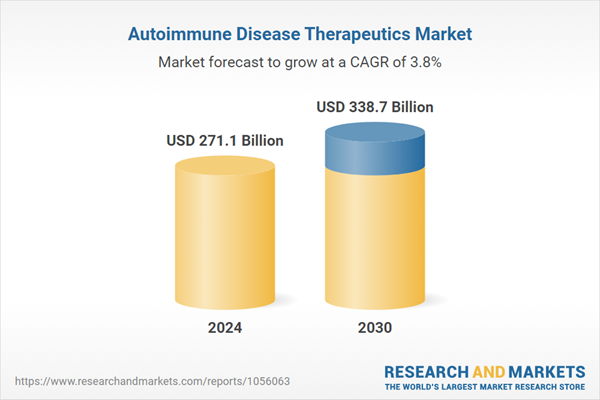
| Estimated Market Value ( USD | $ 271.1 Billion |
| Forecasted Market Value ( USD | $ 338.7 Billion |
| Compound Annual Growth Rate | 3.8% |
| Regions Covered | Global |