Global Anti-Hypertensive Drugs Market - Key Trends and Drivers Summarized
The global market for anti-hypertensive drugs is a major segment within the pharmaceutical industry, driven largely by the widespread prevalence of hypertension, which affects approximately 1.5 billion people worldwide. Despite hypertension being one of the most common chronic diseases globally, there has been a noticeable decline in innovative research on blood pressure mechanisms and new treatments in recent years. This decline is particularly concerning given the rising burden of the disease, especially in low-income countries, coupled with ongoing issues of underdiagnosis and undertreatment in wealthier nations.The demand for anti-hypertensive medications continues to grow, fueled by an aging population, increasing rates of obesity, sedentary lifestyles, and poor dietary choices such as high salt intake. In response, the market is seeing a shift towards the development of new drug therapies that offer better efficacy, reduced side effects, and improved patient compliance, such as combination therapies that incorporate multiple drugs in a single dosage form to simplify treatment regimens. Furthermore, advancements in genomics and biotechnology are paving the way for more personalized approaches to treatment, allowing for medications to be tailored to the genetic profiles of individual patients, enhancing the effectiveness of hypertension management.
Emerging economies are witnessing significant growth in access to anti-hypertensive treatments, driven by expanding middle classes and better health insurance coverage. This expansion is supported by government and healthcare initiatives aimed at increasing public awareness about the risks of high blood pressure and promoting effective management practices. Additionally, the rise of home health monitoring technologies, such as digital blood pressure monitors linked to smartphone apps, is promoting better patient engagement and adherence to treatment by enabling patients to monitor their condition in real-time. However, the industry also faces challenges such as stringent regulatory environments and pressure to reduce drug prices, which are pushing pharmaceutical companies to innovate in cost-effective drug production and distribution methods.
Report Scope
The report analyzes the Anti-Hypertensive Drugs market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Type (Calcium Channel Blockers, ACE Inhibitors, Beta-Adrenergic Blocker, Diuretics, Vasodilators, Other Types).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Calcium Channel Blockers segment, which is expected to reach US$7.2 Billion by 2030 with a CAGR of a 3.2%. The ACE Inhibitors segment is also set to grow at 3.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $6.8 Billion in 2024, and China, forecasted to grow at an impressive 4.7% CAGR to reach $5.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Anti-Hypertensive Drugs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Anti-Hypertensive Drugs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Anti-Hypertensive Drugs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AstraZeneca plc, Bristol-Myers Squibb Company, Celgene Corporation, Eli Lilly and Company, F. Hoffmann-La Roche AG and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 143 companies featured in this Anti-Hypertensive Drugs market report include:
- Actelion Pharmaceuticals Ltd.
- AstraZeneca PLC
- Bayer AG
- Boehringer Ingelheim GmbH
- Daiichi Sankyo Co., Ltd.
- Johnson & Johnson
- Lupin Pharmaceuticals, Inc.
- Merck & Co., Inc.
- Novartis International AG
- Pfizer, Inc.
- Sanofi
- Takeda Pharmaceutical Co., Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Actelion Pharmaceuticals Ltd.
- AstraZeneca PLC
- Bayer AG
- Boehringer Ingelheim GmbH
- Daiichi Sankyo Co., Ltd.
- Johnson & Johnson
- Lupin Pharmaceuticals, Inc.
- Merck & Co., Inc.
- Novartis International AG
- Pfizer, Inc.
- Sanofi
- Takeda Pharmaceutical Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 293 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
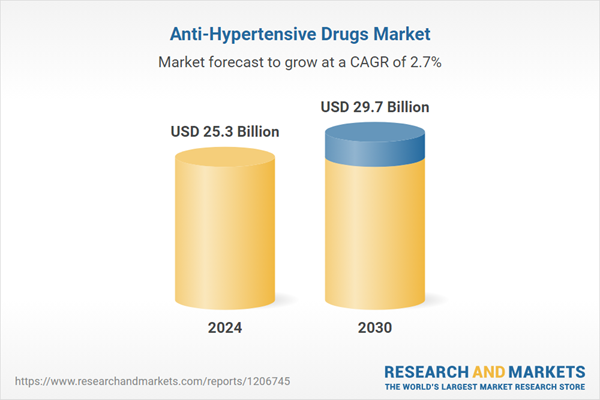
| Estimated Market Value ( USD | $ 25.3 Billion |
| Forecasted Market Value ( USD | $ 29.7 Billion |
| Compound Annual Growth Rate | 2.7% |
| Regions Covered | Global |