Diabetic Retinopathy Treatment - Key Trends and Drivers
Diabetic retinopathy is a severe eye condition that affects individuals with diabetes, characterized by damage to the blood vessels in the retina, which can lead to vision impairment and even blindness if left untreated. The condition progresses through four stages, starting with mild non-proliferative retinopathy, where small areas of swelling occur in the retina's blood vessels, advancing to moderate and severe non-proliferative stages, which involve increasing blockage of blood vessels, and culminating in proliferative retinopathy. In the proliferative stage, new, abnormal blood vessels grow on the surface of the retina and into the vitreous gel inside the eye. These new vessels are fragile and can leak blood, causing severe vision loss. The treatment landscape for diabetic retinopathy has evolved significantly over the years, offering various options to manage and mitigate the condition. Traditional treatments include laser photocoagulation, which seals or shrinks leaking blood vessels, and vitrectomy, a surgical procedure to remove the vitreous gel and blood from leaking vessels. However, pharmacologic interventions, particularly anti-VEGF (vascular endothelial growth factor) injections, have become increasingly prominent in recent years.One of the significant trends in diabetic retinopathy treatment is the increasing use of pharmacologic therapies, particularly anti-VEGF agents like ranibizumab (Lucentis), aflibercept (Eylea), and bevacizumab (Avastin). These injections have become a cornerstone in managing diabetic macular edema and proliferative diabetic retinopathy, providing a less invasive alternative to laser surgery. Anti-VEGF treatments work by inhibiting the action of VEGF, a protein that promotes the growth of abnormal blood vessels in the retina. Clinical trials and real-world studies have demonstrated the efficacy of these agents in reducing retinal swelling, improving vision, and preventing further disease progression. Another emerging trend is the development and adoption of longer-acting treatments and sustained-release drug delivery systems, which aim to reduce the frequency of injections and improve patient compliance. Advances in imaging technologies, such as optical coherence tomography (OCT), have also revolutionized the diagnosis and monitoring of diabetic retinopathy. OCT provides detailed cross-sectional images of the retina, enabling early detection and precise assessment of disease progression, which is crucial for timely and effective treatment. Additionally, artificial intelligence (AI)-driven algorithms are being developed to assist in the detection and grading of diabetic retinopathy, further enhancing diagnostic accuracy and efficiency.
The growth in the diabetic retinopathy treatment market is driven by several factors, including the rising prevalence of diabetes worldwide, increasing awareness about diabetic eye diseases, and advancements in medical technology. The global diabetes epidemic, fueled by factors such as aging populations, sedentary lifestyles, and unhealthy diets, is leading to a higher incidence of diabetic retinopathy, thus expanding the market for treatment options. Additionally, increased efforts by healthcare providers and organizations to educate patients about the importance of regular eye examinations and early intervention are driving demand for diagnostic and therapeutic solutions. Public health campaigns and initiatives by organizations like the American Diabetes Association (ADA) and the International Diabetes Federation (IDF) emphasize the critical need for annual diabetic eye exams, which are essential for early detection and management. Technological advancements, including the development of new pharmacologic agents, innovative drug delivery systems, and improved diagnostic tools, are enhancing treatment efficacy and patient outcomes. Moreover, supportive government policies and increased healthcare expenditure in many regions are facilitating access to advanced diabetic retinopathy treatments. Additionally, the growing presence of specialized retinal care centers and increasing investments in research and development are contributing to the market's expansion.
Report Scope
The report analyzes the Diabetic Retinopathy Treatment market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Type (Non-Proliferative Diabetic Retinopathy, Proliferative Diabetic Retinopathy); Management (Anti-VEGF, Intraocular Steroid Injection, Laser Surgery, Vitrectomy); Distribution Channel (Hospital & Pharmacies, Ambulatory Surgery Centers, Eye Clinics, Other Distribution Channels).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Non-Proliferative Diabetic Retinopathy segment, which is expected to reach US$7.5 Billion by 2030 with a CAGR of a 5.6%. The Proliferative Diabetic Retinopathy segment is also set to grow at 4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.3 Billion in 2024, and China, forecasted to grow at an impressive 8.5% CAGR to reach $2.5 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Diabetic Retinopathy Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Diabetic Retinopathy Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Diabetic Retinopathy Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., Galapagos NV, Genentech, Gilead Sciences Inc., Novartis AG and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Diabetic Retinopathy Treatment market report include:
- Alimera Sciences, Inc.
- Allergan PLC
- Ampio Pharmaceuticals, Inc.
- BCN Peptides S.A.
- Genentech, Inc.
- Glycadia, Inc.
- Kowa Co., Ltd.
- Novartis International AG
- Regeneron Pharmaceuticals, Inc.
- Sirnaomics, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alimera Sciences, Inc.
- Allergan PLC
- Ampio Pharmaceuticals, Inc.
- BCN Peptides S.A.
- Genentech, Inc.
- Glycadia, Inc.
- Kowa Co., Ltd.
- Novartis International AG
- Regeneron Pharmaceuticals, Inc.
- Sirnaomics, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 273 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
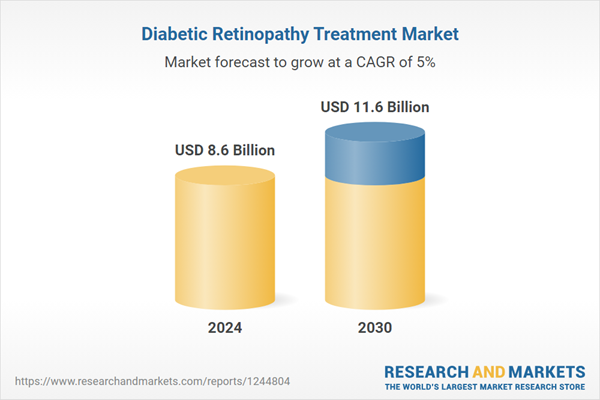
| Estimated Market Value ( USD | $ 8.6 Billion |
| Forecasted Market Value ( USD | $ 11.6 Billion |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |