Report Scope
The aim of this report is to initially conduct a review of the antifungal drugs currently available; explore the issues facing the use of antifungal drugs; and review some of the latest developments of new and innovative antifungal drugs, their technologies, and their intended clinical applications. The key objective is also to conduct and provide an analysis of the market value, growth rates, market shares and market development, and examine the market dynamics and market factors influencing the growth and development of this market.
This report also looks at the challenges and potential threats facing the industry and the factors influencing the market shares of the major market suppliers as well as smaller manufacturers in local markets. The emphasis of this report is to provide the reader with -
- A detailed analysis of the revenues and forecasts for the global antifungal drug market with a more detailed analysis and forecast of the revenues for the global market sub-divided by major market sub-segments by geographic region and selected country.
- A detailed analysis of the global market shares together with a more detailed analysis of the market share by geographic regions and selected country.
In addition, this analysis provides -
- A detailed review of the current products, their indications and availability for all of the market segments was identified.
- Profiles of the individual market sub-segments within the major market segments analyzed and the distinguishing features of each of the market sub-segments.
- A review of the major market opportunities through the recognition of specific high-growth and emerging market opportunities
- Brief descriptions of the historical development for each of the major market segments.
- Profiles of the leading suppliers of antifungal drugs, together with related information about specific products.
The study will allow the reader to -
- Evaluate the effect of strategic factors, such as technology-driven change and industry consolidation, for the antifungal drug market.
- Investigate the current market dynamics that are driving change in the antifungal drug market.
- Assess future growth opportunities in the antifungal drug market.
- Review the main products in each sector and plan a product entry strategy in line with the strengths and weaknesses of the competition. Realize an individual company’s position in the market and gain insight into the future of the market and the opportunities that exist.
The analysis includes the use of charts and graphs measuring product growth and trends within the marketplace. Company-specific information, including sales figures, product pipeline status, and R&D trends, is provided throughout the report.
The Report Includes
- 107 data tables and 30 additional tables
- An updated review of the current and future potential for the global markets for antifungal drugs
- Analyses of the global market trends, with data from 2020, estimates for 2021, and projections of compound annual growth rates (CAGRs) through 2026
- Highlights of key market dynamics (DROs) for antifungal drugs, regulatory scenario, and COVID-19 impact on the progress of this market
- Comparative study of the two primary segments of antifungal medications – prescription antifungal drugs and over the counter (OTC) antifungal drugs - and issues facing the use of these products
- Review of antifungal agents and their classification based on the mechanism of action, approved therapeutic products and relevant patents with their expiration dates
- Information pertaining to R&D efforts, breakthrough therapy innovations, clinical trials of novel drug developments, their technologies and intended clinical applications
- Insight into the growth development strategies of the key market players operating within the global market; their key competitive landscape and company share analysis
- Descriptive company profiles of the major market players, including Dr. Reddy’s Laboratories, Merck & Co., Inc., Sanofi S.A., Pfizer Inc., Bayer AG and Perrigo Company PLC
Table of Contents
Chapter 1 Introduction
- Introduction
- Scope of the Report
- What’s New in the Report
- Methodology and Information Sources
- Primary Data and Information Gathering
- Secondary Data and Information Gathering
- Market Revenue Forecasts
- Geographic Breakdown
- Analyst’s Credentials
- Custom Research
- Related Reports
Chapter 2 Summary and Highlights
- Global Market Revenue Analysis for Antifungal Drugs
- Regional Overview
- Market Segment Review
Chapter 3 Market Dynamics
- Market Dynamics
- Market Drivers
- Market Restraints
Chapter 4 Introduction to Fungi and the Antifungal Drug Market
- Introduction to Fungi
- Classification of Fungi
- Chytridiomycota
- Blastocladiomycota
- Neocallimastigomycota
- Microsporidia
- Ascomycota (Sac Fungi)
- Basidiomycota (Club Fungi)
- Glomeromycota
- Scope of Application: Human Healthcare
- Types and Descriptions of Fungi Diseases in Humans
- Aspergillosis
- Blastomycosis
- Candidiasis
- Coccidioidomycosis (Valley Fever)
- Coccidioidomycosis (Cryptococcosis)
- Dermatophytosis
- Fungal Infections of the Eye
- Histoplasmosis
- Onychomycosis (Fungal Nail Infections)
- Mucormycosis (Zygomycosis)
- Mycetoma
- Paracoccidioidomycosis
- Pneumocystis Pneumonia
- Pseudallescheriasis
- Sporotrichosis
- Rare Fungal Infections and Disorders
- Risk Factors Associated with Human Fungal Infections
- Use of Antibiotics
- Use of Corticosteroids
- Specific Medical Conditions
- Central Line-Associated Bloodstream Infections
- Surgery
- Organ Transplantation
- Environmental Factors
- Hereditary Factors
- Hospitalization
Chapter 5 Common Environmental Fungi
- Molds and the Human Issues Associated with Them
- Why Environmental Mold is a Unique Problem
- Health Risks Due to Environmental Fungi and Mold
- Environmental Molds
- Alternaria
- Aspergillus
- Cladosporium
- Penicillium
- Eurotium
- Rhizopus
- Mucor
- Geotrichum
- Fusarium
- Stachybotrys
- Wallemia
- Trichothecium
- Scopulariopsis Brevicaulis
- Scytalidium Dimidiatum
- Trichoderma
- Paecilomyces Variotii
Chapter 6 Review of Antifungal Agents
- Introduction to Antifungal Agents
- Classification of Antifungal Agents
- Systemic Antifungal Drugs
- Polyene Antibiotics
- Azole Antifungals
- Echinocandins
- Antimetabolite: Flucytosine (5-FC)
- Other Systemic Antifungal Agents
- Topical Antifungal Drugs
- Topical Polyene Antibiotics
- Azoles-Imidazole
- Others
- Naturally Occurring Alternatives
Chapter 7 New and Innovative Antifungals: Current Developments, Clinical Trials and Product Pipelines
- The Need for New and Innovative Antifungals
- Strategic Approach to the Development of New Antifungal Agents
- Identification of Bioactive Compounds
- Nanostructured Antifungals
- Drug Repositioning
- Vaccine Development Against Fungal Diseases
- Artificial Intelligence-Based Platform Technology (FungalAi)
- Emerging Targets and Molecular Scaffolds
- New Antifungal Drugs in Development
- Most Notable Antifungal Compounds
- Specific Company Product Pipelines
- Mycovia Pharmaceuticals
- Amplyx Pharmaceuticals
- F2G
- NovaBiotics Ltd.
- Appili Therapeutics Inc.
- Scynexis Inc.
- Bright Angel Therapeutics
- Valley Fever Solutions
Chapter 8 Global Market for Antifungal Medications
- Introduction
- Market Revenue Analysis
- Global Market Revenue Analysis
- Regional Overview
- Major Developments and Trends in Antifungal Drug Treatment and Technologies
- Prescription Antifungal Drugs
- OTC Antifungal Drugs
- Market Share Analysis
Chapter 9 North American Market for Antifungal Drugs
- Introduction
- Regulatory and Legislative Requirements
- U.S.
- Canada
- Market Dynamics
- Market Drivers
- Market Restraints
- Market Analysis
- North America Market Revenue Analysis
- Regional Overview
- Individual Markets
- Market Share Analysis
Chapter 10 European Market for Antifungal Drugs
- Introduction
- Regulatory and Legislative Requirements
- Mutual Recognition Procedure
- Centralized Procedure
- Decentralized Procedure
- European Suspension of Marketing Authorizations for Oral Ketoconazole
- Over-the-Counter Antifungal Drug Products
- The Russian Federation
- Market Dynamics
- Market Drivers
- Market Restraints
- Market Analysis
- Market Revenue Analysis
- Regional Overview
- Individual Markets
- Market Share Analysis
Chapter 11 Asia-Pacific Market for Antifungal Drugs
- Introduction
- Regulatory and Legislative Requirements
- Japan
- China
- India
- South Korea
- Market Dynamics
- Market Drivers
- Market Restraints
- Market Analysis
- Market Revenue Analysis
- Regional Overview
- Individual Markets
- Market Share Analysis
Chapter 12 Latin American Market for Antifungal Drugs
- Introduction
- Regulatory and Legislative Requirements
- Brazil
- Argentina
- Mexico
- Market Dynamics
- Market Drivers
- Market Restraints
- Market Analysis
- Market Revenue Analysis
- Regional Overview
- Individual Markets
- Market Share Analysis
Chapter 13 Middle Eastern/African Market for Antifungal Drugs
- Introduction
- Regulatory and Legislative Requirements
- South Africa
- Saudi Arabia
- Market Dynamics
- Market Drivers
- Market Restraints
- Market Analysis
- Market Revenue Analysis
- Regional Overview
- Individual Markets
- Market Share Analysis
Chapter 14 Competitive Landscape
- Market Share Analysis
- Recent Developments
Chapter 15 Company Profiles
- Introduction
- Tier 1: Manufacturers of Prescription Pharmaceuticals
- Astellas Pharma Inc.
- Bausch Health Companies Inc.
- Bayer Ag
- Dr. Reddy’S Laboratories Ltd.
- Galderma Pharma S.A.
- Gilead Sciences Inc.
- Glenmark Pharmaceuticals Ltd.
- Janssen Pharmaceuticals Inc. (Johnson & Johnson)
- Leadiant Biosciences Inc.
- Lupin
- Merck & Co., Inc.
- Merz Pharma Gmbh & Co. Kgaa
- Moberg Pharma Ab
- Mylan (Viatris)
- Perrigo Company Plc
- Pfizer Inc.
- Prestige Consumer Healthcare Inc.
- Sanofi S.A.
- Taro Pharmaceutical Industries Ltd.
- Teva Pharmaceuticals Industries Ltd.
Chapter 16 Appendix A
- Tier 1: Large Multinational Companies and Leading Market Share Holders
- Company Contact Details
Chapter 17 Appendix B
- Tier 2: Entrepreneurial Small and Medium-Sized Enterprises
- Company Addresses and Contact Details
Chapter 18 Appendix C
- Research Studies in the Design of Antifungal Vaccine Strategy
Chapter 19 Appendix D
- Government Regulatory Agencies, Professional Organizations and Regulatory Acronyms
Chapter 20 Appendix E
- Acronyms and Terms Commonly Used for Antifungal Drugs and Antifungal Drug Treatments
List of Tables
Summary Table A: Global Market for Antifungal Drugs, by Segment, Through 2026
Summary Table B: Global Market for Antifungal Drugs, by Region, Through 2026
Summary Table C: Global Market for Prescription Antifungal Drugs, by Type, Through 2026
Summary Table D: Global Market for OTC Antifungal Drugs, by Type, Through 2026
Table 1: Statistics on the Ten Most Significant Invasive Fungal Infections
Table 2: Major Clinical Manifestations of Histoplasmosis and Associated Risk Factors
Table 3: Morphologic Features of Fungi and Pseudofungal Infections of Unusual Fungi or of Fungi with Uncertain Etiology
Table 4: Risk Factors Associated with Human Fungal Infections
Table 5: Common Symptoms Associated with Environmental Fungi and Molds
Table 6: Risk Factors Associated with Environmental Fungi and Molds
Table 7: Common Locations of the Sources of Environmental Molds
Table 8: History of Antifungal Therapy
Table 9: Patents on Amphotericin B and Their Expiration Dates
Table 10: Approved Fluconazole Prescription Products
Table 11: Patents on Itraconazole and Their Expiration Dates
Table 12: Patents on Voriconazole and Their Expiration Dates
Table 13: Approved Voriconazole Prescription Products
Table 14: Patents on Posaconazole and Their Expiration Dates
Table 15: Patents on Ketoconazole and Their Expiration Dates
Table 16: Approved Miconazole OTC Products
Table 17: International Clotrimazole Brand Names
Table 18: Approved Generic Prescriptions for Clotrimazole
Table 19: Patents on Caspofungin and Their Expiration Dates
Table 20: Patents on Micafungin and Their Expiration Dates
Table 21: Patents on Anidulafungin and Their Expiration Dates
Table 22: Antifungal Compounds with Novel Targets in Development
Table 23: Global Market for Antifungal Drugs, by Segment, Through 2026
Table 24: Global Market for Antifungal Drugs, by Region, Through 2026
Table 25: Global Market Shares of Antifungal Drugs, by Region, 2020
Table 26: North American Market for Antifungal Drugs, by Segment, Through 2026
Table 27: North American Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 28: North American Market for OTC Antifungal Drugs, by Type, Through 2026
Table 29: North American Market for Antifungal Drugs, by Country, Through 2026
Table 30: U.S. Market for Antifungal Drugs, by Segment, Through 2026
Table 31: U.S. Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 32: U.S. Market for OTC Antifungal Drugs, by Type, Through 2026
Table 33: Canadian Market for Antifungal Drugs, by Segment, Through 2026
Table 34: Canadian Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 35: Canadian Market for OTC Antifungal Drugs, by Type, Through 2026
Table 36: European Market for Antifungal Drugs, by Segment, Through 2026
Table 37: European Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 38: European Market for OTC Antifungal Drugs, by Type, Through 2026
Table 39: European Market for Antifungal Drugs, by Country, Through 2026
Table 40: German Market for Antifungal Drugs, by Segment, Through 2026
Table 41: German Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 42: German Market for OTC Antifungal Drugs, by Type, Through 2026
Table 43: French Market for Antifungal Drugs, by Segment, Through 2026
Table 44: French Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 45: French Market for OTC Antifungal Drugs, by Type, Through 2026
Table 46: U.K. Market for Antifungal Drugs, by Segment, Through 2026
Table 47: U.K. Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 48: U.K. Market for OTC Antifungal Drugs, by Type, Through 2026
Table 49: Italian Market for Antifungal Drugs, by Segment, Through 2026
Table 50: Italian Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 51: Italian Market for OTC Antifungal Drugs, by Type, Through 2026
Table 52: Spanish Market for Antifungal Drugs, by Segment, Through 2026
Table 53: Spanish Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 54: Spanish Market for OTC Antifungal Drugs, by Type, Through 2026
Table 55: Benelux Market for Antifungal Drugs, by Segment, Through 2026
Table 56: Benelux Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 57: Benelux Market for OTC Antifungal Drugs, by Type, Through 2026
Table 58: Scandinavian Market for Antifungal Drugs, by Segment, Through 2026
Table 59: Scandinavian Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 60: Scandinavian Market for OTC Antifungal Drugs, by Type, Through 2026
Table 61: Rest-of-European Market for Antifungal Drugs, by Segment, Through 2026
Table 62: Rest-of-European Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 63: Rest-of-European Market for OTC Antifungal Drugs, by Type, Through 2026
Table 64: Overview of Healthcare in India
Table 65: Asia-Pacific Market for Antifungal Drugs, by Segment, Through 2026
Table 66: Asia-Pacific Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 67: Asia-Pacific Market for OTC Antifungal Drugs, by Type, Through 2026
Table 68: Asia-Pacific Market for Antifungal Drugs, by Country, Through 2026
Table 69: Japanese Market for Antifungal Drugs, by Segment, Through 2026
Table 70: Japanese Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 71: Japanese Market for OTC Antifungal Drugs, by Type, Through 2026
Table 72: Chinese Market for Antifungal Drugs, by Segment, Through 2026
Table 73: Chinese Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 74: Chinese Market for OTC Antifungal Drugs, by Type, Through 2026
Table 75: Indian Market for Antifungal Drugs, by Segment, Through 2026
Table 76: Indian Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 77: Indian Market for OTC Antifungal Drugs, by Type, Through 2026
Table 78: South Korean Market for Antifungal Drugs, by Segment, Through 2026
Table 79: South Korean Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 80: South Korean Market for OTC Antifungal Drugs, by Type, Through 2026
Table 81: Rest of Asia-Pacific Market for Antifungal Drugs, by Segment, Through 2026
Table 82: Rest of Asia-Pacific Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 83: Rest of Asia-Pacific Market for OTC Antifungal Drugs, by Type, Through 2026
Table 84: Latin American Market for Antifungal Drugs, by Segment, Through 2026
Table 85: Latin American Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 86: Latin American Market for OTC Antifungal Drugs, by Type, Through 2026
Table 87: Latin American Market for Antifungal Drugs, by Country, Through 2026
Table 88: Brazilian Market for Antifungal Drugs, by Segment, Through 2026
Table 89: Brazilian Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 90: Brazilian Market for OTC Antifungal Drugs, by Type, Through 2026
Table 91: Argentinian Market for Antifungal Drugs, by Segment, Through 2026
Table 92: Argentinian Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 93: Argentinian Market for OTC Antifungal Drugs, by Type, Through 2026
Table 94: Mexican Market for Antifungal Drugs, by Segment, Through 2026
Table 95: Mexican Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 96: Mexican Market for OTC Antifungal Drugs, by Type, Through 2026
Table 97: Chilean Market for Antifungal Drugs, by Segment, Through 2026
Table 98: Chilean Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 99: Chilean Market for OTC Antifungal Drugs, by Type, Through 2026
Table 100: Rest of Latin American Market for Antifungal Drugs, by Segment, Through 2026
Table 101: Rest of Latin American Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 102: Rest of Latin American Market for OTC Antifungal Drugs, by Type, Through 2026
Table 103: Number of Saudi Arabian Hospitals and Beds, by Sector and Administrative Region
Table 104: Middle Eastern/African Market for Antifungal Drugs, by Segment, Through 2026
Table 105: Middle Eastern/African Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 106: Middle Eastern/African Market for OTC Antifungal Drugs, by Type, Through 2026
Table 107: Middle Eastern/African Market for Antifungal Drugs, by Region/Country, Through 2026
Table 108: Middle Eastern Market for Antifungal Drugs, by Segment, Through 2026
Table 109: Middle Eastern Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 110: Middle Eastern Market for OTC Antifungal Drugs, by Type, Through 2026
Table 111: African Market for Antifungal Drugs, by Segment, Through 2026
Table 112: African Market for Prescription Antifungal Drugs, by Type, Through 2026
Table 113: African Market for OTC Antifungal Drugs, by Type, Through 2026
Table 114: Recent Developments, 2021
Table 115: Astellas Pharma Inc.: Revenues, 2018-2020
Table 116: Astellas Pharma Inc.: Key Developments
Table 117: Bausch Health Companies Inc.: Revenues, by Segment, 2018-2020
Table 118: Bayer AG: Revenues, 2018-2020
Table 119: Dr. Reddy's Laboratories: Revenues, 2018-2020
Table 120: Gilead Sciences Inc.: Revenue & Product, 2018-2020
Table 121: Lupin: Revenues, 2018-2020
Table 122: Merck & Co., Inc.: Total Revenues and Revenues, by Antifungal Product Segment, 2018-2020
Table 123: Perrigo Company PLC 9i: Revenues, 2018-2020
Table 124: Pfizer Inc.: Revenue, by Drug, 2018-2020
Table 125: Prestige Consumer Healthcare Inc.: Revenues, 2018-2020
Table 126: Sanofi S.A.: Revenues, by Segment, 2018-2020
Table 127: Taro Pharmaceutical Industries Ltd.: Revenues, 2018-2020
Table 128: Teva Pharmaceutical Industries Ltd.: Revenues, 2018-2020
Table 129: Tier 1: Major Multinational Medical Device Companies: Contact Details
Table 130: Tier 2: Entrepreneurial Small and Medium-Sized Enterprises Contact Details
Table 131: Studies Conducted in the Design of Antifungal Vaccine Strategy
Table 132: Government Regulatory Agencies, Professional Organizations and Regulatory Acronyms
Table 133: Commonly Used Acronyms and Terms used for Antifungal Drugs and Antifungal Drug Treatments
List of Figures
Summary Figure A: Global Market for Antifungal Drugs, by Segment, 2020-2026
Summary Figure B: Global Market for Antifungal Drugs, by Region, 2019-2026
Summary Figure C: Global Market for Prescription Antifungal Drugs, by Type, 2020-2026
Summary Figure D: Global Market for OTC Antifungal Drugs, by Type, 2019-2026
Figure 1: Schematic of Fungi Classification
Figure 2: Worldwide Distribution of Reported Cases of C. auris, 2018
Figure 3: Number of Reported Cryptococcosis Cases, 1998-2017
Figure 4: Global Burden of HIV-Related Cryptococcal Meningitis, by Region
Figure 5: County-Specific Incidence of Histoplasmosis for the 12 U.S. States for Which Surveillance Data Were Available, 2011-2014
Figure 6: Timeline of Antifungal Drug Development, 1950-2020
Figure 7: Sphingolipid Biosynthetic Pathways
Figure 8: Strategic Approach to the Development of Vaccines for Fungal Infections
Figure 9: Principle Categories of Vaccines for Fungal Infections
Figure 10: Mycovia Pharmaceuticals: Current Product Pipeline
Figure 11: Ibrexafungerp Development Pipeline, 2018-2020
Figure 12: Global Market for Antifungal Drugs, by Segment, 2019-2026
Figure 13: Global Market Shares of Antifungal Drugs, by Segment, 2020
Figure 14: Global Market Shares of Antifungal Drugs, by Region, 2020
Figure 15: Global Market Shares of Antifungal Drugs, by Company, 2020
Figure 16: North American Market for Prescription Antifungal Drugs, by Type, 2020
Figure 17: North American Market for OTC Antifungal Drugs, by Type, 2020
Figure 18: North American Market Shares of Antifungal Drugs, by Company, 2020
Figure 19: Mutual Recognition Procedure
Figure 20: Decentralized Procedur
Figure 21: European Market for Prescription Antifungal Drugs, by Type, 2020
Figure 22: European Market for OTC Antifungal Drugs, by Type, 2020
Figure 23: European Market Shares of Antifungal Drugs, by Company, 2020
Figure 24: Flowchart of New Drug Development and Approval Process in Japan
Figure 25: Timeline of the Standard Process of New Drug Approvals in Japan
Figure 26: Chinese Drug Funding/Reimbursement Approval Procedure
Figure 27: The Chinese Healthcare System
Figure 28: Asia-Pacific Market for Prescription Antifungal Drugs, by Type, 2020
Figure 29: Asia-Pacific Market for OTC Antifungal Drugs, by Type, 2020
Figure 30: Asia-Pacific Market Shares of Antifungal Drugs, by Company, 2020
Figure 31: Argentinian Regulatory Drug Approval Procedure, Standard Review 120-240 Business Days for Approval
Figure 32: Latin American Market for Prescription Antifungal Drugs, by Type, 2020
Figure 33: Latin American Market for OTC Antifungal Drugs, by Type, 2020
Figure 34: Latin American Market Shares of Antifungal Drugs, by Company, 2020
Figure 35: Current Structure of the Healthcare Sectors in Saudi Arabia, 2018
Figure 36: Middle Eastern/African Market for Prescription Antifungal Drugs, by Type, 2020
Figure 37: Middle Eastern/African Market for OTC Antifungal Drugs, by Type, 2020
Figure 38: Middle Eastern/African Market Shares of Antifungal Drugs, by Company, 2020
Figure 39: Global Market Shares of Antifungal Drugs, by Company, 2020
Companies Mentioned
- Amplyx Pharmaceuticals
- Appili Therapeutics Inc.
- Astellas Pharma Inc.
- Bausch Health Companies Inc.
- Bayer Ag
- Bright Angel Therapeutics
- Dr. Reddy’S Laboratories Ltd.
- F2G
- Galderma Pharma S.A.
- Gilead Sciences Inc.
- Glenmark Pharmaceuticals Ltd.
- Janssen Pharmaceuticals Inc. (Johnson & Johnson)
- Leadiant Biosciences Inc.
- Lupin
- Merck & Co., Inc.
- Merz Pharma Gmbh & Co. Kgaa
- Moberg Pharma Ab
- Mycovia Pharmaceuticals
- Mylan (Viatris)
- NovaBiotics Ltd.
- Perrigo Company Plc
- Pfizer Inc.
- Prestige Consumer Healthcare Inc.
- Sanofi S.A.
- Scynexis Inc.
- Taro Pharmaceutical Industries Ltd.
- Teva Pharmaceuticals Industries Ltd.
- Valley Fever Solutions
Table Information
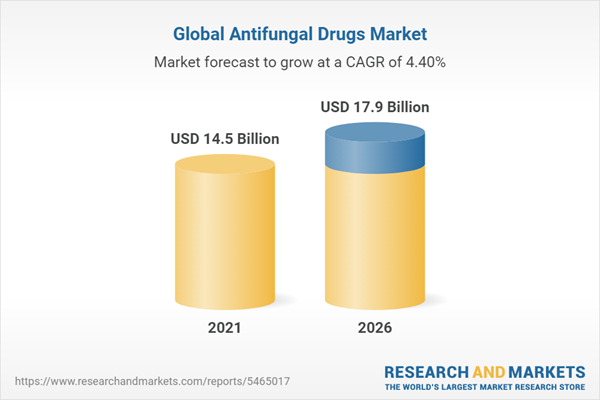
| Report Attribute | Details |
|---|---|
| No. of Pages | 295 |
| Published | November 2021 |
| Forecast Period | 2021 - 2026 |
| Estimated Market Value ( USD | $ 14.5 Billion |
| Forecasted Market Value ( USD | $ 17.9 Billion |
| Compound Annual Growth Rate | 4.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |