The COVID-19 pandemic substantially affected the treatment and diagnostic procedures for cardiac practices owing to delayed diagnosis and multiple postponed appointments, thereby impacting the growth of the studied market for transradial access devices. Furthermore, many studies suggested that people with cardiac diseases were particularly vulnerable to COVID-19, which led to a decrease in footfalls in hospitals and diagnostic centers. For instance, according to the research study published by the NCBI in May 2021, during the COVID-19 pandemic, cardiac procedural activity in England decreased dramatically, with a deficit of about 45,000 procedures. Moreover, a study published in Frontiers in Family Medicine and Primary Care in December 2021 stated that in Germany, despite having symptoms of coronary artery disease (CAD), 9.1% of patients did not see a medical practitioner due to a fear of becoming infected with COVID-19. However, the study's findings also detailed that most patients received adequate medical care during the COVID-19 pandemic for CAD. As a result of the growing availability of cardiac diagnosis and treatments, the market is expected to be favorably influenced in the latter phase, as per the analysis.
The transradial access devices market is growing due to the increasing preference for interventional procedures using radial artery access, the growing prevalence of cardiovascular diseases due to lifestyle disorders, and the increasing use of radial access devices in pediatric patients.
According to the WHO data updated in June 2021, nearly 33.5 million people globally are suffering from atrial fibrillation (AFib), which is the most common type of serious arrhythmia. Moreover, the British Heart Foundation's report published in July 2021 reported that 7.6 million people in the United Kingdom live with heart and circulatory diseases. Further, according to the 2022 AHA report, approximately 244.1 million people were living with IHD around the world. North Africa, the Middle East, Central, and South Asia, and Eastern Europe had the highest prevalence rates of IHD in the world in 2020. With the rise in the aging population worldwide, the number of patients suffering from heart rhythm disorders is likely to increase. This will lead to increased adoption of transradial access, which is expected to drive the growth of the studied market.
A scientific study published by the AHA in August 2021 stated that lower extremity peripheral artery disease (PAD) affects more than 230 million people worldwide and is linked to an elevated risk of several unfavorable clinical outcomes (including cardiovascular diseases like coronary heart disease and stroke and limb outcomes like amputee status). The increased incidence of PAD ultimately boosts the demand for interventional procedures requiring transradial access, augmenting the market’s growth over the forecast period.
However, the major restraining factor for the market's growth is the high costs involved in the placement and maintenance of vascular access devices and the lack of trained professionals, which poses significant challenges.
Transradial Access Market Trends
Catheters Segment is Expected to Witness Growth Over the Forecast Period
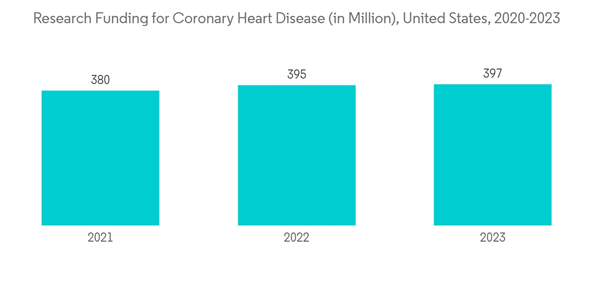
The catheter segment is expected to witness growth over the forecast period. Transradial access (TRA) via the left radial artery is an alternative to traditional transfemoral access for catheter-based procedures that are becoming increasingly relevant in all types of arterial vascular interventions. Cardiac catheterization is a common procedure that can help diagnose heart disease. In some cases, catheterization is also used to treat heart disease by opening blocked arteries with balloon angioplasty and stent placement.Cardiac catheterization helps in the diagnosis of multiple heart diseases, including atherosclerosis, cardiomyopathy, congenital heart disease, heart failure, and heart valve disease. With the growing burden of cardiovascular diseases all over the world, the catheter segment is expected to witness significant growth. As per a February 2022 published update by the CDC, in 2020, the percentage of adults diagnosed with coronary heart disease was 4.6% in the United States. In addition, as of February 2021, approximately 1.5 million heart attacks and strokes occur every year in the United States. Moreover, as per the 2021 PAHO, cardiovascular diseases account for 40.8 million disability-adjusted life years (DALYs) in the Americas. In addition, according to the National Center for Chronic Disease Prevention and Health Promotion data updated in July 2022, coronary heart disease is the most common type of heart disease. Moreover, about 805,000 people in the United States have a heart attack every year. This concerning situation is raising public awareness about disease management and the importance of diagnostics.
Thus, the abovementioned factors are expected to drive catheter segment growth over the forecast period.
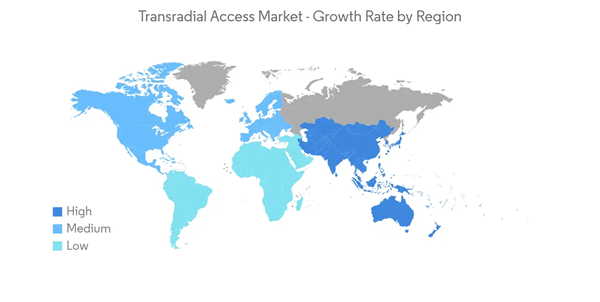
North America Dominates the Market and is Expected to Follow the Same Trend for Few More Years
The North American market is likely to grow with the highest growth rate, owing to factors such as the growing prevalence of CVDs, a rising number of conferences and workshops, and an increase in research and clinical trials for vascular access devices.According to an article published by the CDC in September 2020, heart disease is the leading cause of death in the United States. The same source also reports that every year about 805,000 Americans have a heart attack. As the number of deaths due to heart diseases is increasing, there is a continuous need for the proper treatment and diagnosis of cardiac diseases, since transradial access devices help in the diagnosis and are hence expected to show growth over the forecast period.
According to the CDC updates from February 2022, about 2.1% of children in the United States are in fair or poor health conditions in 2020, and about 3.3% of children aged 5 to 11 in 2020 missed 11 or more days of school in the last 12 months due to some form of illness or injury. Also, as per the above-mentioned source, in the United States, approximately 20.3% of children aged 6 to 11 have obesity, and as obesity is one of the major risk factors for different diseases, the burden of pediatric disease is expected to increase in the country, which would drive growth in the market studied.
The region's transradial access market is expected to benefit from the well-established healthcare industry and rising healthcare expenditure. For instance, according to NHEA data released in December 2021, the United States spent USD 440,000 million on healthcare in 2020. Thus, rising healthcare costs are expected to spur the development of new products such as transradial access devices, which are expected to drive market growth over the forecast period.
Thus, the market is expected to witness a high growth rate over the forecast period due to the factors mentioned above in the North American region.
Transradial Access Market Competitor Analysis
The transradial access market is competitive and fragmented and is inclusive of the big players along with some of the local players in the market. Some of the key players in the market include Becton, Dickinson and Company, Boston Scientific Corporation, Edward Lifesciences Corporation, Medtronic plc, and Terumo Corporation.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Ameco Medical
- Angiodynamics, Inc.
- Becton, Dickinson and Company
- Boston Scientific Corporation
- Edward Lifesciences Corporation
- Medtronic plc
- Merit Medical Systems
- Nipro Medical Corporation
- Oscor Inc
- Smiths Medical
- Teleflex Incorporated
- Terumo Corporation