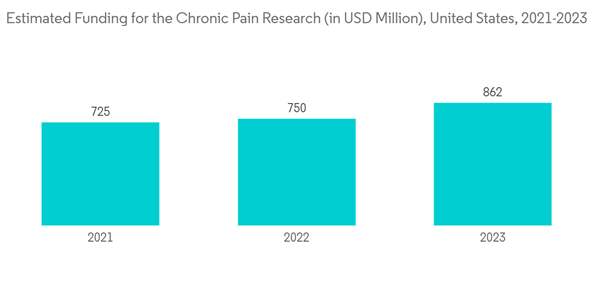
The COVID-19 pandemic impacted the non-opioid pain patch market since pain management during COVID-19 was challenging. There were critical issues related to transport, relocation, and closing down of healthcare centers. According to the PubMed article published in June 2022, most pain management facilities in this pandemic were compelled to close, leaving patients distressed and adding to their suffering. Also, during the pandemic, the government health authorities of the United States, European Union, United Kingdom, India, and the rest of the world stated that all routine chronic pain services went shut because of the pandemic. As a result, the use of non-opioid pain relievers increased. Furthermore, even though import and export activities were restricted during the pandemic, sales of medical supplies (that includes non-opioid patches, too) are likely to increase in the post-COVID-19 situation. The market is estimated to witness significant growth in the future owing to the rise in research and development in non-opioid patches for pain.
Factors such as an increase in the burden of pain-related diseases, a rise in research and development of non-opioid patches, and benefits associated with non-opioid pain patches over conventional medications are expected to drive market growth over the forecast period.
Similarly, cardiovascular disease (CVD) is one of the main causes of death among the adult population globally. Chronic pain occurs in numerous ways, for instance, from trauma, which could include an accident or back strain due to heavy lifting. It is an underlying disease like pancreatitis, spine disease, arthritis, or autoimmune diseases, or it can occur with fibromyalgia or persistent migraines. For instance, the National Spinal Cord Injury Statistical Center (NSCISC) 2021 datasheet accounted for the annual incidence of spinal cord injuries at 60 cases per million. There are numerous risk factors for chronic pain.
Additionally, as per the International Diabetes Federation 2022 update, 537 million adults (20-79 years) are living with diabetes, and 1 in 10 people will be diagnosed with diabetes in 2021. This number is predicted to rise to 643 million by 2030 and 783 million by 2045. The increasing prevalence of diabetes is likely to increase the chance of diabetic neuropathy. Using non-opioid pain patches significantly improves pain and quality-of-life ratings and may allow the tapering of concomitant analgesic therapy in diabetes patients.
Furthermore, the chronic pain associated with rheumatoid arthritis is one of the major factors boosting the market growth since it continuously impacts a person's day-to-day life, and people tend to adopt reliable, easy-going patches for quick pain relief. For instance, as per the 2021 report from the CDC, the prevalence of doctor-diagnosed arthritis is expected to increase in the coming decades. By 2040, an estimated 78.4 million adults aged 18 years and older (25.9% of the projected adult population) will likely suffer from doctor-diagnosed arthritis. Hence, as the arthritis-affected population increases, the chance of adopting non-opioid patches increases, boosting market growth.
Meanwhile, a few other players are conducting extensive R&D by collaborating with educational institutions to introduce new non-narcotic pain patches. For instance, in May 2022, Hisamitsu America Celebrated Salonpas Day with a Free Patch offer from a doctor-recommended OTC Pain Relief Patch Brand. Salonpas was named the number-one doctor-recommended brand of pain relief patches in the United States in 2021. However, the availability of alternative treatments and the low adoption rate of non-opioid pain patch due to unawareness is expected to impede the growth of the non-opioid pain patch market.
Therefore, due to the rise in the prevalence of pain-related diseases coupled with the strategic activities of the key players, the studied market is anticipated to witness significant growth over the forecast period. However, the availability of alternative treatments and low adoption of non-opioid patches for pain due to unawareness are expected to hamper the market growth.
Non-opioid Pain Patch Market Trends
Lidocaine Patches Segment is Expected to Show Significant Market Growth Over the Forecast Years
Lidocaine patches are the most commonly available for treating chronic and acute pain. The factors such as the rise in the prevalence of pain and product launches by the key market players boost the segment growth.Furthermore, various clinical studies are being conducted to demonstrate the efficacy of lidocaine patches in postoperative pain management, which is expected to bolster the market growth over the forecast period. For instance, as per the article published in September 2022 in PubMed, the researchers demonstrated that the 5% lidocaine patch is efficacious in acute postoperative pain after unilateral inguinal hernia repair. Thus, such advantages offered by the lidocaine patches are expected to increase demand and fuel market growth over the forecast period.
Moreover, increasing product launches by key market players are anticipated to boost the market. For instance, in June 2022, Hisamitsu America, a subsidiary of Hisamitsu Pharmaceutical Co., Inc., and the manufacturers of the Salonpas line of pain relief products established the availability of the Salonpas Pain Relieving FLEX Patch Lidocaine 4% featuring thin, flexible, and highly adhesive patch technology. Similarly, in August 2022, Scilex Holding Company, a commercial biopharmaceutical company, focused on developing and commercializing non-opioid therapies for acute and chronic pain patients. It received the fast-track designation for its investigational drug and device product candidate, SP-103. Scilex is developing SP-103 as a non-opioid triple-strength, non-aqueous lidocaine topical system for treating acute LBP. If approved, SP-103 could become the first FDA-approved lidocaine topical product for treating acute LBP. In addition, the increasing burden of diseases, along with the safety profile and efficacy associated with lidocaine patches, are expected to drive the growth of the Non-opioid Pain Patch market during the forecast period.
Therefore, due to the high efficacy of lidocaine patches and the key players' strategic activities, the studied segment is anticipated to witness significant growth over the forecast period.
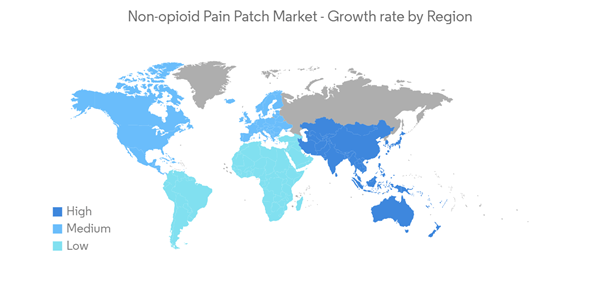
North America is Expected to Witness a Healthy Growth in the Non-opioid Pain Patch Market Over the Forecast Period
North America is expected to witness significant growth in the studied market owing to the rising prevalence of pain-related diseases in the region, as product launches by the key market players in the region are expected to boost the market growth over the forecast period. Chronic pain is one of the primary factors responsible for the market's growth. According to the study published in the JAMA Network in February 2022, it was estimated that one in every five adults in the United States suffers from chronic pain. 1 in 14 adults experienced severe chronic pain. In addition, the countries in North America are increasingly facing scrutiny on the opioid crisis issue from the government authorities, which in turn are expected to benefit from the rise of non-opioid alternatives. Thus, the rising disease burden further pushes the need for effective and quick treatment options, which is expected to propel the market's growth over the forecast period.Similarly, Chronic pain, such as nerve pain, chronic pelvic pain, pain related to endometriosis, carpal tunnel syndrome, pain related to gout, and cancer pain, were studied using a variety of complementary therapies. One of the most common cases in North America is cancer-related pain. Thus, advanced stages of cancer can be more painful. Thus, the rise in cancer cases is expected to augment the market growth over the forecast period. For instance, according to the American Cancer Society 2023 Cancer Statistics, the new cancer cases are estimated to be 1,958,310 in the United States in 2023. This estimation includes 1,010,310 number of males and 948,000 cases of females. The high burden of cancer will likely augment the demand for non-opioid pain patches for reducing cancer pain, thereby boosting the market growth over the forecast period.
In addition, product launches by the key market players are anticipated to boost the market over the forecast period. For instance, in September 2021, Vizuri Health Sciences Consumer Healthcare, Inc. entered into a partnership with a world-renowned surfer, lifestyle coach, and mother, Bethany Hamilton, a partner with the company for the launch of its PainBloc24 ProWomen Pain Relief Patch. Also, in February 2022, NEXGEL, Inc., a provider of ultra-gentle, high-water-content hydrogel products for healthcare and consumer applications, reported the launch of its MEDAGEL Bug Bite Relief Patch. These cooling patches, made in the USA using NEXGEL's soothing hydrogel technology, provide instant relief to irritated skin caused by insect bites.
Thus, due to the high prevalence of pain-related diseases and strategic activities by the key players, the studied region is anticipated to witness significant growth over the forecast period.
Non-opioid Pain Patch Industry Overview
The market is highly fragmented because numerous companies are working to help a large global population suffering from chronic pain. Various market players use different technologies to develop products that facilitate effective and prolonged drug release to increase consumer base and product penetration. Furthermore, the market is highly competitive, with fierce competition between existing players and new entrants. The global non-opioid pain patch market players are Endo Pharmaceuticals, Hisamitsu Pharmaceutical, Sanofi, Sorrento Therapeutics (SCILEX Pharmaceuticals), and TEH SENG Pharmaceutical Mfg. Co., Ltd, Teikoku Seiyaku Co. Ltd, Teva Pharmaceuticals, and Veridian Healthcare.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Endo Pharmaceuticals Inc.
- Hisamitsu Pharmaceutical Co. Inc.
- Sanofi
- Sorrento Therapeutics (SCILEX Pharmaceuticals)
- TEH SENG Pharmaceutical Mfg. Co., Ltd
- Teikoku Seiyaku Co. Ltd.
- Teva Pharmaceuticals Industries Ltd.
- Averitas Pharma
- Sparsha Pharma International Pvt Ltd
- Veridian Healthcare