The global active implantable medical devices (AIMD) market is experiencing significant growth, driven by technological advancements, an aging population, and the rising prevalence of chronic diseases. AIMDs, such as pacemakers and neurostimulators, are critical for monitoring and treating conditions, particularly in geriatric patients.
Market Drivers
- Rising Prevalence of Chronic Diseases in the Aging Population: The growing incidence of chronic conditions, such as cardiovascular diseases, diabetes, and neurological disorders, among older adults is a primary driver of the AIMD market. These devices play a vital role in managing complex healthcare needs, improving patient outcomes, and enhancing quality of life. The increasing elderly population globally amplifies demand for AIMDs, as healthcare providers seek advanced solutions to address age-related health challenges.
- Technological Advancements and AI Integration: Innovations in AIMD technology, including the integration of artificial intelligence (AI) for patient-specific diagnostics, are propelling market growth. AI-enabled devices enhance monitoring precision and treatment efficacy, driving adoption. Ongoing research and development, along with expanded indications for existing AIMDs, further fuel innovation, positioning the market for sustained growth.
- Supportive Government Policies and Funding: Regulatory frameworks, such as the Republic of Korea's Act on Fostering the Medical Devices Industry and Supporting Innovative Medical Devices, are fostering AIMD development. Government funding and increased awareness of AIMD benefits are encouraging investment in research and product launches, supporting market expansion.
Market Restraints
- High Costs and Regulatory Challenges: The high cost of developing and manufacturing AIMDs, coupled with stringent regulatory requirements, poses challenges to market growth. Compliance with diverse global standards increases production costs and timelines, potentially limiting accessibility in cost-sensitive markets.
- Limited Awareness in Emerging Markets: Despite growing demand, limited awareness of AIMD benefits in certain regions, particularly in developing economies, may hinder market penetration. Educating healthcare providers and patients about the advantages of AIMDs remains a critical challenge.
Geographical Outlook
- North America (United States): The U.S. is expected to hold the largest market share, driven by the high prevalence of cardiovascular diseases. The aging population, representing 17% of the U.S. population in 2022 per World Bank data, further boosts demand for AIMDs. Technological advancements, such as Abbott Laboratories FDA-approved AVEIR dual-chamber pacemaker in July 2023, enhance market growth by offering innovative solutions for heart rhythm disorders.
- Asia-Pacific: Countries like India, with a high burden of chronic diseases (21% of the elderly affected, per the National Library of Medicine), are seeing increased demand for AIMDs. Supportive regulatory frameworks and investments in healthcare infrastructure are driving growth in this region.
Key Developments (2023-2025)
- July 2023: Abbott Laboratories received U.S. FDA approval for its AVEIR dual-chamber pacemaker, expanding treatment options for patients with abnormal heart rhythms in the U.S.
Market Outlook
The AIMD market is poised for robust growth through 2025, driven by the rising burden of chronic diseases, technological advancements, and supportive policies. The U.S. leads due to its aging population and high disease prevalence, while Asia-Pacific shows strong potential. Challenges such as high costs and regulatory complexities must be addressed to sustain growth. Industry players are expected to focus on AI integration and innovative product launches to meet evolving healthcare demands.Key Benefits of this Report:
- Insightful Analysis: Gain detailed market insights covering major as well as emerging geographical regions, focusing on customer segments, government policies and socio-economic factors, consumer preferences, industry verticals, and other sub-segments.
- Competitive Landscape: Understand the strategic maneuvers employed by key players globally to understand possible market penetration with the correct strategy.
- Market Drivers & Future Trends: Explore the dynamic factors and pivotal market trends and how they will shape future market developments.
- Actionable Recommendations: Utilize the insights to exercise strategic decisions to uncover new business streams and revenues in a dynamic environment.
- Caters to a Wide Audience: Beneficial and cost-effective for startups, research institutions, consultants, SMEs, and large enterprises.
What do businesses use our reports for?
Industry and Market Insights, Opportunity Assessment, Product Demand Forecasting, Market Entry Strategy, Geographical Expansion, Capital Investment Decisions, Regulatory Framework & Implications, New Product Development, Competitive Intelligence.Report Coverage:
- Historical data from 2020 to 2024 & forecast data from 2025 to 2030
- Growth Opportunities, Challenges, Supply Chain Outlook, Regulatory Framework, and Trend Analysis
- Competitive Positioning, Strategies, and Market Share Analysis
- Revenue Growth and Forecast Assessment of segments and regions including countries
- Company Profiling: Strategies, Products, Financial Information, and Key Developments among others
Active Implantable Medical Devices (AIMD) Market Segmentation:
By Product
- Ventricular Assist Devices
- Neurostimulators
- Implantable Cardiac Pacemakers
- Cochlear Implants
- Others
By End-User
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Specialty Centers
- Home Care Settings
By Geography
- North America
- South America
- Europe
- Middle East and Africa
- Asia Pacific
Table of Contents
Companies Mentioned
- Sonova Holding AG
- Medtronic
- Abbott
- Lifetech Scientific
- Boston Scientific Corporation
- Cochlear Ltd.
- MED-EL
- Biotronik SE & Co. KG
- LivaNova PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 146 |
| Published | August 2025 |
| Forecast Period | 2025 - 2030 |
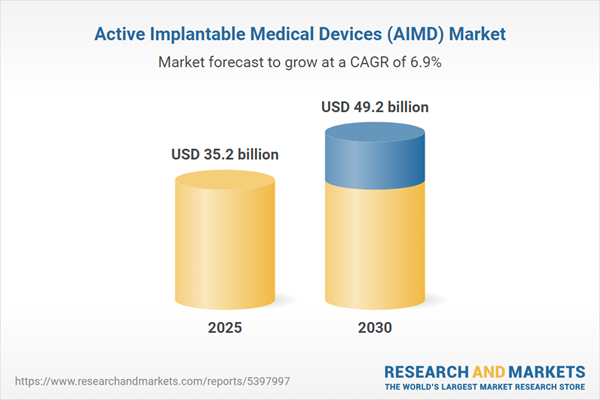
| Estimated Market Value ( USD | $ 35.2 billion |
| Forecasted Market Value ( USD | $ 49.2 billion |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |