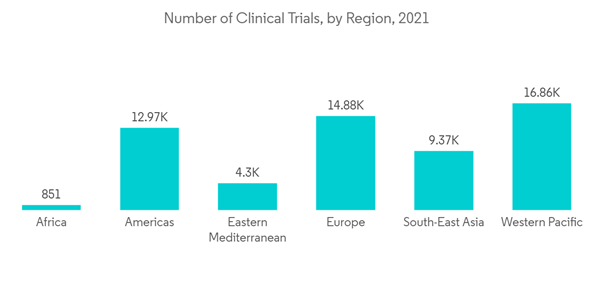
The COVID-19 pandemic had a big effect on the contract research organization (CRO) market because clinical trials were still going on to find effective treatments and vaccines for the SARS-CoV-2 virus. For example, in January 2021, ICON PLC and Pfizer Inc. and BioNTech announced that they would work together to develop the investigational COVID-19 vaccine program that Pfizer and BioNTech were working on.As a part of this program, ICON worked at more than 153 sites in Europe, South Africa, the United States, and Latin America to recruit 44,000 participants for clinical trial studies. Besides, the number of mergers and acquisitions (M&A) among CROs saw an uptick in 2021, despite the COVID-19 pandemic. Clinical Trials Arena said in April 2022 that there were 50 completed M&A deals last year, which is 21 more than in 2020, when there were only 29.There were 20 completed M&A CRO deals in the first half of 2021, with 30 such deals in the second half. After a pandemic, CROs will be able to use the lessons they learned from COVID-19 and adopt new technologies to improve data management, reduce staffing needs, and make patient monitoring easier.For example, a Frontiers Media S.A. article from April 2022 says that the use of AI technology helps SMEs turn COVID-19 challenges into chances to improve their performance and their chances of staying in business.Thus, due to the increased focus on vaccine development against COVID-19, there was an increase in clinical trials along with the adoption of emerging technologies, which boosted the CRO market.
The growth of the market is due to more money being spent on research and development, more R&D activities being outsourced, and more clinical trials.Pharmaceutical companies' bottom lines continue to be affected by things like patents that expire and let generic competitors enter the market and strict requirements from regulators.According to the Congressional Research Service update, by September 2022, global R&D spending will have significantly increased since 2000, rising from USD 675 billion to USD 2.4 trillion by 2020. Furthermore, according to the UNESCO Institute for Statistics (UIS), in 2022, nearly USD 1.7 trillion will have been spent globally on R&D, which is a record high. Approximately 10 nations receive 80% of the money spent. Countries have committed to significantly boosting public and corporate R&D spending as well as the number of researchers by 2030 as part of the Sustainable Development Goals. Hence, with strong investments in R&D activities and a robust pipeline of therapeutics under development, the CRO market is expected to witness significant growth over the forecast period.
The growth of the market is also being driven by the fact that CROs are offering more and more services and that collaborations are becoming more popular. For example, in September 2022, the Australian CRO for biotechs, Avance Clinical, grew into North America by buying the CRO partner company C3 Research Associates. This made it easy for biotech clients to move from early-phase studies to later-phase studies.Thermo Fisher Scientific, Inc. also bought Pharmaceutical Product Development, LLC, a company that helps the biotech and biopharma industries, in December 2021.Thus, due to these factors, the market is expected to register significant growth over the forecast period.
However, the shortage of skilled professionals is expected to restrain market growth.
Contract Research Organization Market Trends
The Early-phase Development Services Segment is Poised to Witness Robust Growth During the Forecast Period
With more and more drug patents expiring, generic versions of drugs are becoming more of a threat, and drug companies are under pressure to make up for the money they lose because of generics.R&D costs are going up because drug molecules are getting more complicated and regulations are getting stricter.Additionally, leading CROs have developed significant expertise in the field of early-phase development, and CROs are leveraging this expertise to offer highly efficient and accurate early-phase development services. CROs are also allowing small and midsize firms to enter the complex drug development process without significant investment in capital equipment. According to the March 2021 published article by Anju Life Sciences Software, high data quality, better safety decisions, reduced trial operation costs, and faster study execution are making early phase trials more successful. Thus, the early-phase development services segment is poised to register significant growth.Additionally, growing company partnerships and increased clinical trial demand are expected to drive the segment. Furthermore, in June 2021, Cancer Research UK and Aleta Biotherapeutics signed an agreement to progress the early-phase clinical development of ALETA-001, their CAR-T cell engager. ALETA-001 has been developed to benefit people with B-cell lymphoma and leukemia whose disease has progressed after receiving CD19 CAR-T cell therapy. Thus, such factors are expected to boost the early-phase development services segment over the forecast period.
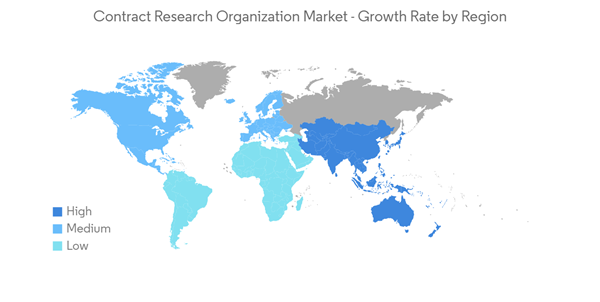
North America Captured the Largest Market Share and is Expected to Retain its Dominance During the Forecast Period
Major pharmaceutical companies are outsourcing R&D and clinical trials because of changes in reimbursement and competition from generic drugs. This is helping the growth of the CRO market in North America.The growth of the market in the region is due to the fact that pharmaceutical companies are spending more money on research and development to create new medicines.For instance, the article published by the National Library of Medicine in July 2022 reported that in 2021, overall pharmaceutical expenditures in the United States were USD 576.9 billion, an increase of 7.7% compared to 2020. Thus, the increasing pharmaceutical expenditure in the country is also bolstering the studied market's growth.Additionally, according to Federal Research and Development (R&D) Funding: FY2022, funding for R&D is concentrated in a few federal departments and agencies. In FY 2021, five agencies received 93.0% of total federal R&D funding, with the Department of Health and Human Services receiving 27.6%. The largest dollar increases in R&D funding would be made to Health and Human Services, up to USD 7.7 billion (17.8%).
By and large, the presence of key players in the region and strategic collaborations by these companies are driving market growth. For instance, in February 2021, Parexel International partnered with Neogenomics to enable the application of Neogenomics' real-world genomics data in oncology clinical trials, thereby leading to increased efficiency in patient matching and optimizing trial design, site selection, clinical development, and translational research. Thus, the high R&D investment and initiatives by key players in the expansion of the product portfolio are expected to boost the development of new drugs, thus increasing the demand for outsourcing services.
Contract Research Organization Industry Overview
The market for contract research organizations is fragmented because there are many companies around the world and in different regions. The competitive landscape includes an analysis of international as well as local companies that hold market shares and are well known, including IQVIA, ICON PLC, Charles River Laboratories, Envigo, and Laboratory Corporation of America Holdings (Labcorp), among others.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Charles River Laboratories
- Envigo
- ICON PLC
- IQVIA
- Laboratoty Corporation of America Holdings (Labcorp)
- MedPace Holdings Inc.
- PSI CRO AG
- Thermo Fisher Scientific
- SGS SA
- Syneos Health
- WuXi Pharmatech
- Axcent Advanced Analytics (A3)
- BIO Agile Therapeutics