Since the start of the epidemic, the United States has recorded 79 million cases of COVID-19. Over 960,000 people have died in the United States because of COVID-19. The average number of new COVID-19 cases per day has decreased to 40,000, approximately 30,000 less than it was a week ago, according to CDC data. In January 2021, after the FDA had authorized COVID-19 vaccines for emergency use the month before, people in the U.S. began to be vaccinated. By March, the daily number of new infections had steeply declined, and April through June saw those numbers go down even further. But by July, the arrival and spread of the delta coronavirus variant sparked another surge in cases of COVID-19.
Diabetes is associated with many health complications. Comparing the population with and without diabetes, those with diabetes have a 300% increased risk of being hospitalized and, thus, incur more healthcare expenses compared to non-diabetic people. People with diabetes face a higher chance of experiencing serious complications from COVID-19. Doctors and the government are asking diabetes patients to check their glucose levels continuously. In general, people with diabetes are more likely to experience severe symptoms and complications when infected with a virus. According to the AACE (American Association of Clinical Endocrinologists), recent studies have shown that of those hospitalized for severe disease, 22.2-26.9% reported living with diabetes. Diabetes and high glucose levels are associated with increased complications, respiratory failure, and mortality in hospitalized patients with coronavirus.
The pandemic also highlighted opportunities for continuing and expanding innovations in the delivery of diabetes care, through virtual consultations between healthcare providers and people with diabetes, and the use of diabetes technology. Crisis management has created unprecedented interest in remote care from both patients and providers and removed many long-standing regulatory barriers. Thus, the COVID-19 outbreak increased the diabetes care device market's growth.
US Diabetes Devices Market Trends
Growing Diabetes and Obesity Population in the United States
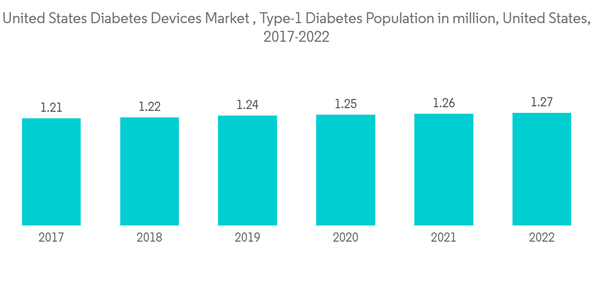
In the United States, the prevalence of diabetes has increased dramatically during the last two decades, a fact driven by the increased prevalence of obesity and lifestyle changes. Diabetes ranks among the fastest-growing chronic diseases in the United States. About 1.75 million US citizens are diagnosed with diabetes every year. The country also has the highest obesity population, which is a prominent cause of type-2 diabetes.In the United States, several innovations by startups, like Glooko, OneDrop, Verily, Vacate, Insulet, Noom, Bigfoot Biomedical, Virta Health, Diabeloop, and Orgenesis, have launched in the market. The market for diabetes care devices is expected to experience steady growth in the coming years due to the greater prevalence of obesity due to less physical activity, unhealthy food habits, and other lifestyle factors. The growing awareness among people regarding advanced diabetes devices is improving their adaptability to diabetes devices. Leading manufacturers are focusing on technological innovations and the development of advanced products to gain a substantial share of the market. There has been a significant rise in insulin delivery system technology, ranging from insulin injections to insulin pumps. Technological innovations and advancements offer many conveniences for measuring blood glucose levels. Abbott and Dexcom received FDA approval to use continuous glucose monitoring in US hospitals for coronavirus-affected people. Dexcom began shipping CGMs to hospitals in April 2020. It plans to make 100,000 sensors for hospitals at low costs. The company has been planning to donate 10,000 phones and CGM readers to hospitals to scan those sensors.
Therefore, owing to the increasing prevalence of diabetes, the studied market is anticipated to witness growth over the analysis period.
Continuous Glucose Monitoring Segment is Expected to Witness Highest Growth Rate Over the Forecast Period
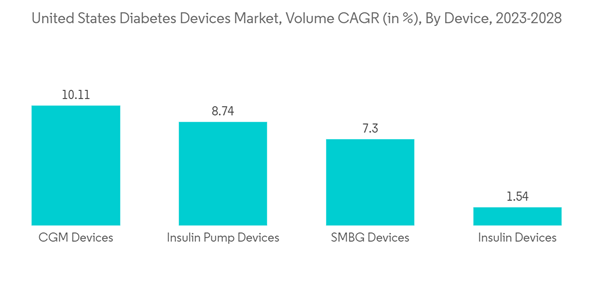
The continuous glucose monitoring segment recorded more than USD 2 billion in revenue in the current year, which is expected to further increase with a CAGR of more than 9% during the forecast period.Continuous glucose monitoring sensors use glucose oxidase to detect blood sugar levels. Glucose oxidase converts glucose to hydrogen peroxidase, which reacts with the platinum inside the sensor, producing an electrical signal to be communicated to the transmitter. Sensors are the most important part of continuous glucose monitoring devices. Technological advancements to improve the accuracy of the sensors are expected to drive segment growth during the forecast period.
The COVID-19 pandemic emphasized the need for good glycemic control in patients with diabetes, in large part because most observational studies have reported that poorly controlled diabetes is associated with a higher risk of hospitalization and death from a viral illness. The frequency of monitoring glucose levels depends on the type of diabetes, which varies from patient to patient. Type-1 diabetic patients need to check their blood glucose levels at regular intervals to monitor their blood glucose levels and adjust their insulin dosing accordingly. The current CGM devices show a detailed representation of blood glucose patterns and tendencies compared to a routine check of glucose levels at set intervals. Furthermore, the current continuous glucose monitoring devices can either retrospectively display the trends in the levels of blood glucose by downloading the data or give a real-time picture of glucose levels through receiver displays.
The use of CGM by type-1 diabetic patients is very low as compared to type-2 diabetic patients. However, type-1 diabetic patients spend nearly twice as much on these devices as type-2 diabetics do. The newest CGM models, the Abbott Freestyle Libre and the Dexcom G6, overcame many technical barriers. However, people with type-2 diabetes have not used CGM as widely as they might due to their high costs and uncertainties regarding their necessity and efficacy. Moreover, to lower the cost burden of diabetic patients in the United States, Abbott introduced a new, lower-priced category with Libre, at around USD 75 to USD 150 each month (2 sensors that last 14 days each). Continuous glucose monitoring devices are becoming cheaper with the advent of new technologies like cell phone integration, which is likely to drive segment growth during the forecast period.
US Diabetes Devices Industry Overview
The United States diabetes care devices market is moderately fragmented, with few significant and generic players. The mergers and acquisitions between the players, like the acquisition of TypeZero Technologies by Dexcom, are paving the way for automated insulin delivery. The acquisition has sent Dexcom ahead on its way in the race to create an artificial pancreas system, rather than merely offering a boost to the continuous glucose monitoring devices market. There have been constant innovations driven by manufacturers such as Abbott and Medtronic, while also adhering to organic growth strategies, which is evident from the R&D spending of these companies.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Becton and Dickenson
- Medtronic
- F. Hoffmann-La Roche AG
- Insulet Corporation
- Abbott
- Dexcom
- Tandem
- Johnson & Johnson
- Ypsomed Holding
- Novo Nordisk
- Sanofi
- Eli Lilly and Company