The COVID-19 pandemic positively impacted the European Dipeptide Peptidase 4 (DPP-4) Inhibitors Market. Diabetes and uncontrolled hyperglycemia are risk factors for poor outcomes in patients with COVID-19 including an increased risk of severe illness or death. People with diabetes have a weaker immune system, the COVID-19 complication aggravates the condition, and the immune system gets weaker very fast. In the current crisis, type 2 diabetes patients are at much higher risk.
According to the diabetes category, the estimated cost per hospital admission during the first wave of COVID-19 in Europe ranged from EUR 25,018 for type 2 diabetes patients in good glycemic control to EUR 57,244 for type 1 diabetes patients in poor glycemic control, reflecting a higher risk of intensive care, ventilator support, and a longer hospital stay. The estimated cost for patients without diabetes was EUR 16,993. The expected total direct expenditures for COVID-19 secondary care in Europe were 13.9 billion euros. Diabetes treatment thus accounted for 23.5% of total expenditures.
Dipeptidyl peptidase 4 (DPP-4) inhibitors are a class of medicine that lower high blood glucose levels and are used in the treatment of type 2 diabetes. DPP4 inhibitors increase insulin and GLP-1 secretion and are commonly prescribed for people suffering from type 2 diabetes. The use of DPP4 inhibitors in patients with COVID-19 with or even without type 2 diabetes offers a simple way to reduce the virus entry and replication into the airways and to hamper the sustained cytokine storm and inflammation within the lung in patients diagnosed with COVID-19 infection. Technological advancements have increased over the period leading to several modifications either in the DPP-4 Inhibitor drugs or the formulations being developed.
Therefore, owing to the aforementioned factors the studied market is anticipated to witness growth over the analysis period.
Europe Dipeptide Peptidase 4 (DPP-4) Inhibitors Market Trends
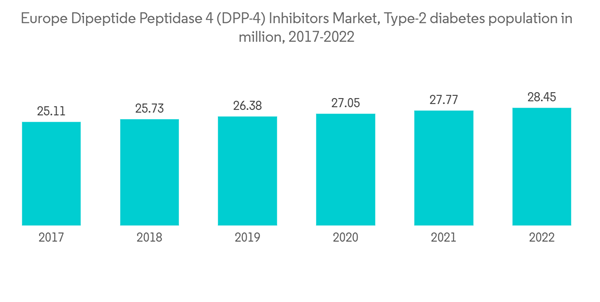
Increasing diabetes prevalence
The diabetes population in the European region is expected to rise by about 5% over the forecast period.According to the IDF 2021 report, about 1 in 11 adults include diabetes in Europe which accounts for approximately 61 million. The overall diabetes expenditure in Europe was USD 189 billion. These figures indicate that approximately 19.6% of global expenditure is spent on diabetes in Europe.
The European region witnessed an alarming increase in the prevalence of diabetes in recent years. Patients with diabetes require many corrections throughout the day to maintain nominal blood glucose levels, such as oral anti-diabetic medication or ingestion of additional carbohydrates by monitoring their blood glucose levels. The rate of newly diagnosed Type 1 and Type 2 diabetes cases is seen to increase, mainly due to obesity, unhealthy diet, and physical inactivity. The rapidly increasing incidence and prevalence of diabetic patients and healthcare expenditure are indications of the increasing usage of diabetic drugs.
Dipeptidyl peptidase 4 (DPP-4) inhibitors are effective in treating type 2 diabetes, as they maintain blood glucose levels through the degradation of incretin peptides, glucagon-like peptide 1 (GLP-1), and glucose-dependent insulinotropic polypeptide. Oral Anti-Diabetic Drugs are available internationally and are recommended for use when treatment escalation for type 2 diabetes is required along with lifestyle management. They are typically the first medications used in treating type 2 diabetes due to their wide range of efficacy, safety, and mechanisms of action. They help diabetes patients control their condition and lower the risk of diabetes complications. These agents present the advantages of easier management and lower cost, so they became an attractive alternative to insulin with better acceptance, which enhances adherence to the treatment.
The government and the companies are working towards better diabetes management. For instance, in the United Kingdom, the National Service Framework (NSF) program is improving services by setting national standards to improve service quality and tackle variations in care. The Association of British HealthTech Industries (ABHI) launched a diabetes section, enabling diabetes technology companies to work together in the first forum of its kind.
Owing to the rising rate of obesity, growing genetic factors for type-2 diabetes, the increasing prevalence, and the factors above, the market will likely continue to grow.
Germany held the highest market share in the European Dipeptide Peptidase 4 (DPP-4) Inhibitors Market in the current year
Germany held the highest market share of about 21.6% in the European Dipeptide Peptidase 4 (DPP-4) Inhibitors Market in the current year.Diabetes is a significant health problem and one of the tremendous challenges for healthcare systems all over Germany. The prevalence of known type 1 & 2 diabetes in the German adult population is very high, along with many patients not yet diagnosed with the disease. Due to an aging population and unhealthy lifestyle, the prevalence of type 2 diabetes is expected to increase steadily over the next few years. High-quality care, including adequate monitoring, control of risk factors, and active self-management, are the key factors for preventing complications in German patients with type 2 diabetes.
The disease's growing incidence, prevalence, and progressive nature encouraged the development of new drugs to provide additional treatment options for diabetic patients. Non-insulin treatments, used as first-line therapies for patients with type 2 diabetes, currently capture more than half the sales in the anti-diabetic market. Over the past decade, two important classes entered this market: dipeptidyl peptidase-4 inhibitors (DPP-4) and sodium-glucose cotransporter-2 inhibitors (SGLT-2). These agents work in various ways to reduce blood sugar levels in people with type 2 diabetes. Some stimulate insulin secretion by the pancreas, and others improve the responsiveness of cells to insulin or prevent glucose production by the liver. Others slow the absorption of glucose after meals. Also, the use of oral anti-diabetes drugs is rising because new-generation oral drugs, such as DPP-4 and SGLT-2, reduce the rate of CV risk in diabetes patients.
According to the German Diabetes Centre (DDZ), about 8.5 million people in Germany are affected by diabetes. The number of people with type 2 diabetes in Germany will continue to increase over the next twenty years. German law requires public plans to cap out-of-pocket health care costs and to cover all medically necessary treatment, including insulin. Germany is one of the developed countries with advanced healthcare facilities. Moreover, reimbursement and pricing policies are highly regulated, which drives the market. The roll-out of many new products, increasing international research collaborations in technology advancement, and increasing awareness about diabetes among the people are some market opportunities for the players in the German market.
Europe Dipeptide Peptidase 4 (DPP-4) Inhibitors Industry Overview
The European Dipeptide Peptidase 4 (DPP-4) Inhibitors Market is consolidated, with a few major manufacturers like Eli Lilly, AstraZeneca, Merck, Boehringer Ingelheim, Novartis, etc., gaining presence in major countries. At the same time, the remaining market comprises other local or region-specific manufacturers.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Merck and Co.
- AstraZeneca
- Bristol Myers Squibb
- Novartis
- Takeda Pharmaceuticals
- Eli Lilly
- Boehringer Ingelheim