The COVID-19 pandemic impacted several interventional cardiology (IC) and electrophysiology (EP) operations to be performed globally. For instance, according to an article published in PLOS ONE in September 2022, during the COVID-19 pandemic, there was a reduction in percutaneous coronary intervention (PCI) volumes and higher risk-adjusted mortality. COVID-19-positive patients experienced significantly worse outcomes. Thus, the COVID-19 pandemic had an adverse impact on the market's growth in its preliminary phase.
Although the COVID-19 pandemic significantly impacted market growth in the initial phases, with the help of government initiatives, innovation, and digital health, the market has recovered. For instance, according to an article published by Current Cardiology Reports in March 2022, remote telehealth monitoring, telehealth clinic visits, and patient education through social media were implemented and showed benefits to those at higher risk of cardiovascular events.
Additionally, robotic PCI emerged as a promising strategy in experienced centers during the pandemic, and it facilitated procedural distancing, minimized exposure risk, and decreased PPE cost. Hence, with better access to healthcare access and the implementation of telemedicine during the pandemic, the market was expected to gain its growth pace over the forecast period.
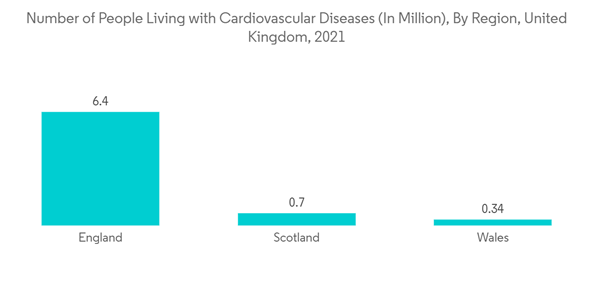
Furthermore, the major factors for the growth of the interventional cardiology devices market include the growing prevalence of coronary artery diseases (CADs), technological advancements in interventional cardiology devices, and the growing demand for minimally invasive treatment globally. For instance, according to the British Heart Foundation's Report published in July 2022, 7.6 million people in the United Kingdom lived with heart and circulatory diseases in 2021, out of which 4 million were men and 3.6 million were women. Therefore, the increasing burden of heart disease is anticipated to boost the usage of interventional cardiology devices, in turn driving the market growth over the forecast period.
Moreover, market players are competing to launch advanced products for better treatment and have invested in research and development programs to improve their existing product lines and launch new product portfolios. For instance, in February 2022, Cardiovascular Systems, Inc. partnered with Innova Vascular, Inc. (Innova) to develop a full line of novel thrombectomy devices. Similarly, in July 2021, ORIGIN SC and ORIGIN NC, two high-performance balloons for vessel preparation, were launched by MedAlliance for patients suffering from life-threatening coronary and peripheral arterial disease. Such development is expected to drive the growth of the market over the forecast period.
Hence, as per the aforementioned factors, such as the growing prevalence of coronary artery diseases (CADs), technological advancements in interventional cardiology devices, and the growing demand for minimally invasive treatment globally, the interventional cardiology devices market is likely to grow over the forecast period. However, a stringent regulatory scenario for manufacturing interventional cardiology devices and the availability of effective first-line treatments are expected to restrain the market's growth.
Interventional Cardiology Devices Market Trends
Drug-eluting Stents Segment Is Expected to Witness Significant Growth Over The Forecast Period
Drug-eluting stents are coated with a slow-release medication to help prevent blood clots from forming in a stent. This helps the artery remain smooth and open, ensuring good blood flow. These stents are usually used for the lower extremities, and they provide results that are better than self-expanding and balloon-expanding stents.Thus, the growing adoption of advanced cardiovascular treatments and the increased funding and technological advancements in the field of cardiology are expected to be the major drivers for the growth of the segment. For instance, according to the study published by the National Institute of Health in February 2022, to reduce in-stent restenosis, coronary drug-eluting stents were developed. Drug combinations having several effects or use for various actions are both delivered through the drug-eluting stent. Thus, the benefits of the drug-eluting stent to a delivered combination of drugs are expected to increase its adoption and are anticipated to boost the segment's growth.
Companies are introducing new and more sophisticated drug-eluting stent systems, which are anticipated to significantly support the growth of the segment. For instance, in August 2021, SINOMED, a medical device company, reported commercial implantation of the HT Supreme Drug-eluting Stent (DES) at the University Hospital Galway, in partnership with the National University of Ireland Galway, marking the start of its European launch.
Similarly, in May 2022, Medtronic plc received U.S. FDA approval for the Onyx Frontier drug-eluting stent (DES). As the latest evolution in the Resolute DES family, Onyx Frontier DES leverages the same stent platform as Resolute Onyx DES, with an enhanced delivery system. Likewise, in January 2021, Boston Scientific received FDA approval for its Synergy Megatron Drug Eluting stent system, which is designed for the treatment of large coronary arteries near the aorta.
Thus, owing to the aforementioned factors, such as the growing adoption of advanced cardiovascular treatments and the increased funding and technological advancements in the field of cardiology, the drug-eluting stents segment is expected to register healthy growth over the forecast period.
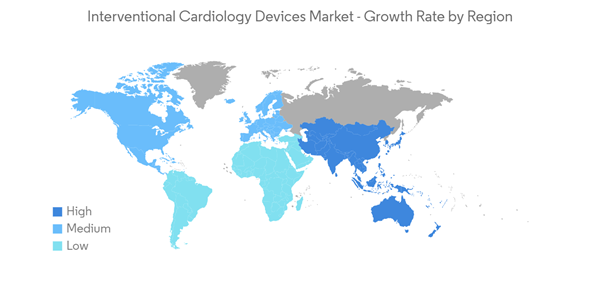
North America is Expected to Show a Significant Growth in Market Over the Forecast Period
North America is expected to hold significant market growth throughout the forecast period. The favorable government initiatives for product development and high patient awareness levels, coupled with an increasing target patient population and an increase in the geriatric population, are expected to be some of the prominent drivers for the growth of the interventional cardiology devices market over the coming years in the region. For instance, according to the World Bank update in 2022, the estimated population of ages 65 and above was 10.5 million in 2021. Thus, with the high burden of target disease and an increase in the geriatric population, the market is expected to increase the demand for interventional cardiology devices, thereby driving the market's growth.Additionally, a few key market players in the region are developing novel products and technologies to compete with existing products, while others are acquiring and partnering with other companies trending in the market. For instance, in June 2021, Abbott received the United States Food and Drug Administration's approval for its XIENCE family of stents for its one-month dual-antiplatelet therapy (DAPT) labeled for high bleeding risk (HBR) patients in the United States. Similarly, in March 2021, the U.S. FDA approved, one of the first in the world, non-surgical heart valve to treat pediatric and adult patients with a native or surgically repaired right ventricular outflow tract (RVOT), the part of the heart that carries blood out of the right ventricle to the lungs.
Thus, owing to the aforementioned factors such as favorable government initiatives for product development, and high patient awareness levels, coupled with an increasing target patient population and an increase in the geriatric population, the market studied is anticipated to witness steady growth in the North American region over the forecast period.
Interventional Cardiology Devices Industry Overview
The interventional cardiology device market is moderately concentrated in nature due to the presence of companies operating globally and regionally. The companies in the market include Abbott Laboratories, B. Braun Melsungen AG, Biosensors International Ltd, Biotronik, Boston Scientific Corporation, Cardinal Health Inc., Cook Medical Inc., Medtronic PLC, and Terumo Medical Corporation, among others.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- B. Braun Melsungen AG
- Biosensors International Group Ltd
- Biotronik SE and Co. KG
- Boston Scientific Corporation
- Cardinal Health Inc.
- Cook Medical Inc.
- Medtronic PLC
- Terumo Medical Corporation
- Edwards Lifesciences Corporation
- Koninklijke Philips N.V.
- Nano Therapeutics Pvt. Ltd.