The sudden outbreak of COVID-19 had an optimistic effect on the global anti-viral therapeutics market’s growth. The strong market growth due to the COVID-19 pandemic was mainly attributed to the increasing focus of major market players on developing COVID-19 drugs. For instance, in January 2022, Pharma major Lupin launched the antiviral drug Molnupiravir in India under the brand name Molnulup for treating COVID-19 adults in the country. Furthermore, Emcure Pharmaceuticals received an Emergency Use Authorisation (EUA) approval from the Drug Controller General of India (DCGI) for launching Molnupiravir to treat mild COVID-19. The company plans to launch the oral drug under the brand Lizuvira in the Indian market. Thus, the market responded to the effect quickly and changed in a way that accelerated market growth.
Similarly, product launches are another factor boosting the growth of the market. For instance, in November 2021, Pfizer Inc. announced its investigational COVID-19 oral antiviral candidate, PAXLOVID, and claimed to significantly reduce hospitalization and mortality rate based on an interim analysis of the Phase 2/3 EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients) randomized, double-blind study of non-hospitalized adult patients with COVID-19, who are at high risk of progressing to severe illness. The anti-viral therapeutics market is expected to witness an upsurge mainly due to the increasing patient population in the world. According to WHO, globally, 38.4 million people were living with HIV at the end of 2021. An estimated 0.7% of adults aged 15-49 years worldwide live with HIV; however, the epidemic's burden continues to vary considerably between countries and regions. Thus, with an increase in HIV cases, the need for its therapy increased, leading to market growth.
Moreover, intense research & development pipelines of the major players and increasing product launches are the key drivers in the growth of the antiviral therapeutics market. For instance, in April 2021, Atriva Therapeutics GmbH received USD 12.05 million (EUR 11.4 million) in federal funding. The company will use the funds to advance its drug ATR-002, which is currently in a Phase II clinical study called RESPIRE. Anti-viral therapeutics are commercially available for diseases like influenza, hepatitis C, chickenpox, papilloma, and AIDS.
Thus, the abovementioned factors are impacting the market growth for the anti-viral therapeutics market. However, the high cost of anti-viral drug treatment is expected to restrain the market growth.
Anti-Viral Therapeutics Market Trends
Influenza Anti-viral Drugs Segment Expected to Hold Significant Market Share over the Forecast Period
Influenza is an infection of a part of the respiratory system, i.e., the nose, throat, and lungs. The primary reason for the growth of the influenza anti-viral drugs segment is the global prevalence of influenza and the increasing number of drug launches. According to the data published by the Centers for Disease Control and Prevention, in February 2022, the common cold causes 22 million school days to be missed each year in the United States. Also, in the United States, almost 1 billion people have colds each year. Furthermore, according to the estimates published by the Centers for Disease Control and Prevention (CDC), 1,675 (0.2%) of 818,939 respiratory specimens tested by United States clinical laboratories were found positive for an influenza virus from September 28, 2020, to May 22, 2021.To cater to the significant unmet needs in the market, players are focusing on research & development with potential candidates. For instance, in August 2022, Genentech, a member of the Roche Group, announced that the United States FDA had approved a supplemental New Drug Application (sNDA) for Xofluza (baloxavir marboxil) for the treatment of acute, uncomplicated influenza in otherwise healthy children aged between 5 years to less than 12 years, who have been symptomatic for no more than 48 hours. The novel launch of products from this strong pipeline is anticipated to propel market growth over the forecast period.
Therefore, the factors mentioned above are expected to drive segmental growth in the market during the forecast period.
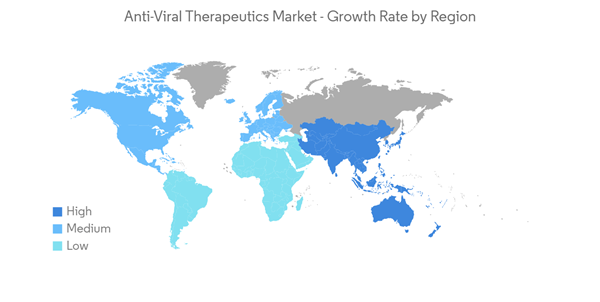
North America Expected to Continue Market Dominance over the Forecast Period
North America is anticipated to continue to dominate the anti-viral therapeutics market over the forecast period due to the presence of well-established market players along with the growing prevalence of HIV and other viral infections. For instance, according to an article published by HIV.gov, in October 2022, approximately 1.2 million people in the United States had HIV. About 13% are unaware of the disease and need HIV testing. Furthermore, the market’s growth in the North American region is also attributed to the increasing investments in research & development by major players and robust drug approvals by the US Food and Drug Administration (FDA) in recent years. For instance, in December 2021, the FDA considered authorizations for Pfizer's PAXLOVID and Merck & Co.'s MOLNUPIRAVIR, the first two oral COVID-19 antivirals.Similarly, product approval is another factor driving the growth of the market. For instance, in December 2021, Pfizer Inc. announced that the United States FDA had authorized the emergency use of PAXLOVID to treat mild-to-moderate COVID-19 in adults and pediatric patients with positive results of direct SARS-CoV-2 viral testing and who are at high-risk for progression to severe COVID-19.
Thus, owing to the abovementioned factors, the North American region is expected to drive market growth during the forecast period.
Anti-Viral Therapeutics Market Competitor Analysis
The anti-viral therapeutics market is moderately competitive. Some of the major players in the anti-viral therapeutics market are AbbVie Inc., GlaxoSmithKline PLC, Bristol-Myers Squibb Company, Johnson & Johnson, Merck and Co. Inc. The anti-viral therapeutics market is a fast-growing market. The major players in the market are adopting organic and inorganic strategies to survive and expand. Moreover, the key players are focusing on increasing US Food and Drug Administration (FDA) approvals and product launches to leverage current and future market opportunities.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- AstraZeneca
- Boehringer Ingelheim
- Bristol-Meyrs Squibb Company
- Cipla Inc.
- Dr. Reddy's Laboratories Ltd
- F. Hoffmann-La Roche Ltd
- GlaxoSmithKline PLC
- Johnson & Johnson
- Merck & Co. Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi SA
- Gilead Sciences Inc.