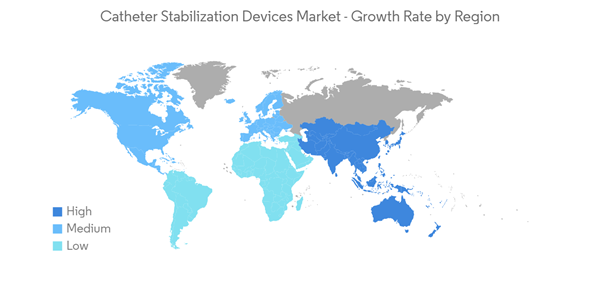
Over the next few years, the market for catheter stabilization devices is expected to grow at a CAGR of 6.5%.
The pandemic has significantly impacted the market for catheter stabilization devices. Due to the increasing number of COVID-19 patients, the demand for intubation, ventilators, and ventilation stabilization supplies such as catheter securement devices increased to a great extent. However, the number of surgical procedures decreased during the pandemic, which reduced the demand for catheter stabilization devices during the COVID-19 pandemic. For instance, according to the NCBI research article published in June 2021, it was observed that post-lockdown daily out-patient visits were reduced to four patients per day in India, and around 77.0% of the professionals did not perform even a single surgical procedure during the pandemic. Furthermore, only urgent surgeries were allowed during the pandemic, and most cardiac procedures were canceled or postponed due to the diversion of resources toward the affected patients. Thus, the pandemic imposed a slight negative impact on the market. However, with the declining cases of COVID-19 and the resumption of surgical procedures, the market started to gain momentum and is anticipated to maintain the upward trend over the forecast period.
The growing burden of lifestyle diseases, the increasing demand for minimally invasive surgeries, and growing awareness pertaining to catheter-related complications are the major factors propelling market growth. For instance, according to the data published by the European Heart Network in 2021, in the European Union, more than 60.0 million people live with cardiovascular disease, and close to 13.0 million new cases of cardiovascular disease are diagnosed every year. Similarly, according to a study published by the British Heart Foundation in July 2021, there were more than 7.5 million people with heart disease in the United Kingdom and nearly 170,000 deaths each year, an average of 460 fatalities each day or one death every three minutes, in the United Kingdom due to cardiovascular disorders (CVDs). As catheter stabilization devices are frequently used in cardiovascular surgeries, the high prevalence of cardiovascular diseases indicates that the demand for such devices is expected to increase over the forecast period, thereby spurring market growth.
In addition, several market players are implementing strategic initiatives, which are contributing to market growth. For instance, in June 2021, STARBOARD MEDICAL, INC., a medical technology company focused on improving the securement of patient catheters, announced that the United States Patent and Trademark Office (USPTO) recently issued another United States Patent, No. 11,020,566 B2, for Starboard's Clik-FIX catheter securement line. The awarded patent further strengthens the company's intellectual property portfolio, expanding its patent claims and solidifying new design improvements. Therefore, such a scenario may create new opportunities in the market, which may favor market growth over the forecast period.
However, the preference for alternative products and product recalls may likely restrain market growth over the forecast period.
The major factors fueling the segment's growth are the implementation of strategic initiatives by the market players. The increasing number of chronic kidney diseases leading to kidney transplants and surgical procedures is also propelling segment growth. For instance, as per the research report published by the Health Resources & Services Administration (HRSA) in March 2022, around 24,670 kidney transplants were performed in the United States in 2021. As urinary catheter stabilization devices are used in renal transplantation procedures, an increasing number of transplantation procedures indicates a significant demand for these devices. In addition, several market players are engaged in implementing strategic initiatives, which is also boosting segment growth. For instance, in November 2021, CATHETRIX, an innovative developer of urinary (Foley) smart catheter fixations, presented its new catheter stabilizer for the prevention of UTIs and accidental Foley catheter extractions at MEDICA 2021, which took place between November 15-18 in Düsseldorf, Germany.
Thus, owing to the increasing transplantation procedures and product launches, the segment is expected to witness significant growth over the forecast period.
Key product launches, a high concentration of market players or manufacturers' presence, acquisitions and partnerships among major players, and increasing cases of chronic diseases in the United States are some of the factors driving the growth of the catheter stabilization devices market in the country. Several market players are launching new products, which is boosting market growth. For example, in March 2021, Argon Medical Devices introduced the Skater mini-loop drainage catheter, which is placed through the skin using image guidance as a minimally invasive procedure for removing or draining unwanted fluid collection. Thus, due to the above-mentioned factors, the market is expected to witness significant growth over the forecast period in the North American region.
This product will be delivered within 2 business days.
The pandemic has significantly impacted the market for catheter stabilization devices. Due to the increasing number of COVID-19 patients, the demand for intubation, ventilators, and ventilation stabilization supplies such as catheter securement devices increased to a great extent. However, the number of surgical procedures decreased during the pandemic, which reduced the demand for catheter stabilization devices during the COVID-19 pandemic. For instance, according to the NCBI research article published in June 2021, it was observed that post-lockdown daily out-patient visits were reduced to four patients per day in India, and around 77.0% of the professionals did not perform even a single surgical procedure during the pandemic. Furthermore, only urgent surgeries were allowed during the pandemic, and most cardiac procedures were canceled or postponed due to the diversion of resources toward the affected patients. Thus, the pandemic imposed a slight negative impact on the market. However, with the declining cases of COVID-19 and the resumption of surgical procedures, the market started to gain momentum and is anticipated to maintain the upward trend over the forecast period.
The growing burden of lifestyle diseases, the increasing demand for minimally invasive surgeries, and growing awareness pertaining to catheter-related complications are the major factors propelling market growth. For instance, according to the data published by the European Heart Network in 2021, in the European Union, more than 60.0 million people live with cardiovascular disease, and close to 13.0 million new cases of cardiovascular disease are diagnosed every year. Similarly, according to a study published by the British Heart Foundation in July 2021, there were more than 7.5 million people with heart disease in the United Kingdom and nearly 170,000 deaths each year, an average of 460 fatalities each day or one death every three minutes, in the United Kingdom due to cardiovascular disorders (CVDs). As catheter stabilization devices are frequently used in cardiovascular surgeries, the high prevalence of cardiovascular diseases indicates that the demand for such devices is expected to increase over the forecast period, thereby spurring market growth.
In addition, several market players are implementing strategic initiatives, which are contributing to market growth. For instance, in June 2021, STARBOARD MEDICAL, INC., a medical technology company focused on improving the securement of patient catheters, announced that the United States Patent and Trademark Office (USPTO) recently issued another United States Patent, No. 11,020,566 B2, for Starboard's Clik-FIX catheter securement line. The awarded patent further strengthens the company's intellectual property portfolio, expanding its patent claims and solidifying new design improvements. Therefore, such a scenario may create new opportunities in the market, which may favor market growth over the forecast period.
However, the preference for alternative products and product recalls may likely restrain market growth over the forecast period.
Catheter Stabilization Devices Market Trends
Urinary Catheters Securement Devices Segment is Expected to Witness Significant Growth Over the Forecast Period
A catheter securement device, or retaining device, is a product that is used to secure an indwelling urinary catheter. It is essential to prevent urethral trauma. A catheter securement device, or retention device, is a product used to secure an indwelling urinary catheter. It is essential to avoid trauma to the urethra. Failure to secure the catheter can lead to urethral damage and inflammation. This could cause pain, discomfort, and a high risk of infection for the patient.The major factors fueling the segment's growth are the implementation of strategic initiatives by the market players. The increasing number of chronic kidney diseases leading to kidney transplants and surgical procedures is also propelling segment growth. For instance, as per the research report published by the Health Resources & Services Administration (HRSA) in March 2022, around 24,670 kidney transplants were performed in the United States in 2021. As urinary catheter stabilization devices are used in renal transplantation procedures, an increasing number of transplantation procedures indicates a significant demand for these devices. In addition, several market players are engaged in implementing strategic initiatives, which is also boosting segment growth. For instance, in November 2021, CATHETRIX, an innovative developer of urinary (Foley) smart catheter fixations, presented its new catheter stabilizer for the prevention of UTIs and accidental Foley catheter extractions at MEDICA 2021, which took place between November 15-18 in Düsseldorf, Germany.
Thus, owing to the increasing transplantation procedures and product launches, the segment is expected to witness significant growth over the forecast period.
North America is Expected to Witness Considerable Growth Over the Forecast Period
North America is expected to witness significant market growth over the forecast period. The major factors contributing to the market's growth are the growing number of surgical procedures, the presence of strong market players, and improved healthcare infrastructure. For instance, according to the CDC article in September 2021, heart diseases are the leading cause of death in the United States. The same source also reports that every year about 805,000 Americans have a heart attack. As the mortality due to heart diseases is low in the region, there is a continuous need for treatment devices for such diseases. For instance, according to the CDC report in 2021, more than 600,000 women in the United States have a hysterectomy every year. All these procedures require catheter stabilization devices, owing to which an increasing number of chronic diseases and surgical procedures are expected to boost market growth in the region.Key product launches, a high concentration of market players or manufacturers' presence, acquisitions and partnerships among major players, and increasing cases of chronic diseases in the United States are some of the factors driving the growth of the catheter stabilization devices market in the country. Several market players are launching new products, which is boosting market growth. For example, in March 2021, Argon Medical Devices introduced the Skater mini-loop drainage catheter, which is placed through the skin using image guidance as a minimally invasive procedure for removing or draining unwanted fluid collection. Thus, due to the above-mentioned factors, the market is expected to witness significant growth over the forecast period in the North American region.
Catheter Stabilization Devices Market Competitor Analysis
The catheter stabilization devices market is moderately competitive with the presence of several global and international players. The key players are adopting different growth strategies to enhance their market presence, such as partnerships, agreements, collaborations, new product launches, geographical expansions, mergers, and acquisitions. Some of the major players in the market are B. Braun SE, Baxter, Becton, Dickinson & Company, Centurion Medical Products, and 3M, among others.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
1 INTRODUCTION
4 MARKET DYNAMICS
5 MARKET SEGMENTATION (Market Size by Value - USD Million)
6 COMPETITIVE LANDSCAPE
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M
- B. Braun SE
- Baxter
- BD (C.R. Bard)
- ConvaTec Group PLC
- Medline Industries Inc.
- Medtronic PLC
- Merit Medical Systems
- TIDI Products LLC
- Tractus Vascular
- Argon Medical Devices
- Levity Products Incorporated
- CATHETRIX
Methodology

LOADING...