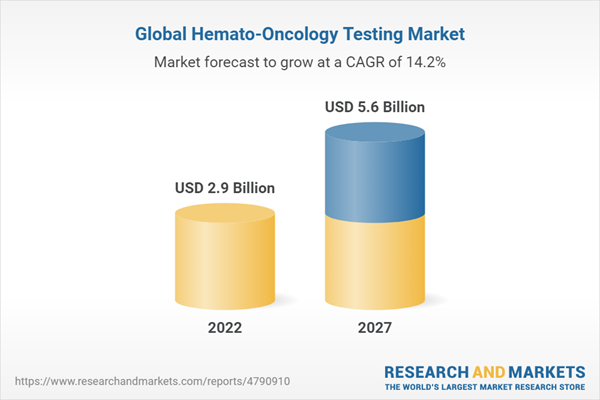
The global Hemato oncology testing market is projected to reach USD 5.6 billion by 2027 from an estimated USD 2.9 Billion in 2022, at a CAGR of 14.2%. The growth in this market is attributed to the growing incidence of hematologic cancer, increasing collaborations, and the increasing number of conferences on personalized medicine.
The Services segment accounted for the highest growth rate in the Hemato oncology testing market, by product and services type, during the forecast period
On the basis of products & services type, the global hemato oncology testing market is segmented into services and assay kits.
In 2021, The services segment accounted for the largest share of the global hemato oncology testing market in 2021. This can be attributed to the increase in the number of hematologic cancer significantly. Hence the the patient needs continuous monitoring and testing during the treatment, the increasing collaboration between the companies for conducting the clinical trails on the hematologic cancers requires the hemato oncology testing products and the raising number of the diagnostic centers for diagenetic testing procedures is expected to boost the demand for the the services segment in the hemato oncology testing product market .
Lymphoma segment accounted for the highest CAGR by cancer type, during the forecast period
By cancer type, the global leukemia, lymphoma, and other cancers market. In 2021, the lymphoma segment dominated the global market. This can be attributed to factor such as the increasing prevalence lymphoma and the rising geriatric population across the globe is supporting the increasing incidence of the lymphoma cases which is a major driving factor for this market.
The PCR segment accounted for the highest growth rate in the Hemato oncology testing market, by technology type, during the forecast period
Based on type, the Hemato oncology testing market technology is segmented into PCR, IHC, NGS, cytogenetics, and other technologies.
In 2021, the PCR segment dominated hemato oncology testing market. Factors supporting the growth of the segment is the wide use of this technology owing to its ease of use and easy availability of assay kits.
The Asia-Pacific market is expected to grow at the highest CAGR during the forecast period.
The Hemato oncology testing market is segmented into - North America, Europe, the Asia Pacific and ROW. The Hemato oncology testing market in several Asia-Pacific countries is expected to witness high growth during the forecast period. Market growth will be driven by the rising geriatric population, increasing demand for quality healthcare, and the growing focus on cancer biomarkers by various stakeholders in their respective healthcare systems.
A breakdown of the primary participants referred to for this report is provided below:
- By Company Type: Tier 1-45%, Tier 2-35%, and Tier 3- 45%
- By Designation: C-level-32%, Director-level-20%, and Others-48%
- By Region: North America-35%, Europe-28%, Asia Pacific-25%, ROW - 12%
Lists of Companies Profiled in the Report:
- Abbott Laboratories (US)
- F. Hoffman-La Roche (Switzerland)
- QIAGEN (Germany)
- Thermo Fisher Scientific (US)
- Illumina (US)
- Bio-Rad Laboratories (US)q
- MolecularMD (Ireland)
- ArcherDX (US)
- ARUP Laboratories (US)
- Asuragen (US)
- Invivoscribe (US)
- Adaptive Biotechnologies (US)
- Amoy Diagnostics (China)
- ELITechGroup (France)
- Vela Diagnostics (Singapore)
- Gentronix (UK)
- BioIVT (US)
- SAGA Diagnostics (Sweden)
- Olink (Sweden)
- Cancer Diagnostics (US)
Research Coverage
This report studies the Hemato oncology testing market based on the type of product, cancer, technology and region. The report also studies factors (such as drivers, restraints, opportunities, and challenges) affecting market growth. It analyzes the opportunities and challenges in the market and provides details of the competitive landscape for market leaders. Furthermore, the report analyzes micromarkets with respect to their individual growth trends and forecasts the revenue of the market segments with respect to five main regions (and the respective countries in these regions.
Key Benefits of Buying the Report:
The report will help market leaders/new entrants by providing them with the closest approximations of the revenue numbers for the overall Hemato oncology testing market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to better position their business and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, opportunities and challenges.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Adaptive Biotechnologies.
- Amoy Diagnostics Co. Ltd
- Archerdx, Inc.
- Arup Laboratories Inc.
- Asuragen, Inc. a Bio-Techne Brand
- Bio-Rad Laboratories, Inc.
- Bioivt
- Cancer Diagnostics
- Elitechgroup
- F. Hoffman-La Roche Ltd.
- Gentronix
- Illumina, Inc.
- Invivoscribe, Inc.
- Molecularmd (Subsidiary of Icon plc)
- Olink
- Qiagen N.V.
- Saga Diagnostics
- Thermo Fisher Scientific, Inc.
- Vela Diagonostics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 141 |
| Published | November 2022 |
| Forecast Period | 2022 - 2027 |
| Estimated Market Value ( USD | $ 2.9 Billion |
| Forecasted Market Value ( USD | $ 5.6 Billion |
| Compound Annual Growth Rate | 14.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |