Global Specimen Validity Testing Market - Key Trends and Drivers Summarized
Why Is Specimen Validity Testing Becoming Essential in Drug Screening?
Specimen validity testing (SVT) is becoming increasingly essential in drug screening programs to ensure the accuracy, reliability, and integrity of test results. As drug abuse and misuse continue to pose significant challenges in workplaces, law enforcement, and sports, the need for robust drug screening methods that can detect tampering, adulteration, substitution, and dilution of specimens is on the rise. Specimen validity testing, which includes pH checks, specific gravity tests, creatinine levels, and the detection of oxidants and other adulterants, plays a crucial role in identifying attempts to manipulate test outcomes. This ensures that drug testing programs remain effective and credible, safeguarding public safety, organizational compliance, and employee health.How Are Technological Advancements Shaping the Specimen Validity Testing Market?
Technological advancements in specimen validity testing methods, equipment, and reagents are significantly enhancing the accuracy, speed, and reliability of drug screening programs. The development of integrated, automated, and rapid testing platforms is gaining traction, enabling laboratories and clinics to streamline specimen validity testing and reduce turnaround times. Innovations in point-of-care (POC) testing devices, remote testing solutions, and digital data management systems are expanding the application scope of specimen validity testing beyond traditional laboratory settings to include workplace testing, remote healthcare, and field testing. Additionally, advancements in reagent formulations, such as enzymatic and chemical reagents, are improving the detection of newer adulterants and masking agents, supporting the growth of the specimen validity testing market.Which Market Segments Are Leading the Growth of the Specimen Validity Testing Industry?
Product types include reagents, assay kits, POC devices, and testing instruments, with assay kits holding the largest market share due to their widespread use in clinical and forensic laboratories. Test types comprise validity tests for pH, specific gravity, creatinine, oxidants, nitrites, and glutaraldehyde, with pH and specific gravity tests being the dominant segments due to their effectiveness in detecting specimen tampering. End-use sectors span clinical laboratories, forensic laboratories, workplaces, sports organizations, and law enforcement, with clinical laboratories leading the market due to the high demand for accurate and reliable drug screening. Geographically, North America and Europe are the largest markets for specimen validity testing, driven by stringent regulatory requirements, while Asia-Pacific is expected to witness rapid growth due to increasing awareness and adoption of drug testing programs.What Are the Key Drivers of Growth in the Specimen Validity Testing Market?
The growth in the specimen validity testing market is driven by several factors, including rising demand for drug testing in workplaces, law enforcement, and clinical settings, technological advancements in specimen validity testing methods and equipment, and the increasing focus on ensuring the accuracy and integrity of drug screening programs. The need to detect tampering, adulteration, substitution, and dilution in drug tests is driving the demand for specimen validity testing solutions across various sectors. Technological innovations in integrated, automated, and remote testing platforms, coupled with advancements in reagent formulations, POC devices, and digital data management, are enhancing the effectiveness, speed, and marketability of specimen validity testing, supporting market growth. The expansion of specimen validity testing applications in healthcare, sports, government, and military sectors, along with the growing emphasis on regulatory compliance, standardization, and quality assurance, is creating new opportunities for market players. Additionally, the focus on developing non-invasive, portable, and digital validity testing solutions for diverse environments is further propelling the growth of the specimen validity testing market.Report Scope
The report analyzes the Specimen Validity Testing market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Offering (Products, Services); Type (Laboratory Testing, Rapid / POC Testing); End-Use (Workplaces, Drug Screening Laboratories, Drug Rehabilitation Centers, Pain Management Centers, Law Enforcement Agencies, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Specimen Validity Testing Products segment, which is expected to reach US$2.9 Billion by 2030 with a CAGR of 4.4%. The Specimen Validity Testing Services segment is also set to grow at 6.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $866.8 Million in 2024, and China, forecasted to grow at an impressive 4.7% CAGR to reach $683.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Specimen Validity Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Specimen Validity Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Specimen Validity Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Acm Global Laboratories, Alfa Scientific Designs, Inc., Clinical Reference Laboratory, Inc., Express Diagnostics Int'l, Inc., Premier Brands of America, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Specimen Validity Testing market report include:
- Acm Global Laboratories
- Alfa Scientific Designs, Inc.
- Clinical Reference Laboratory, Inc.
- Express Diagnostics Int'l, Inc.
- Premier Brands of America, Inc.
- Quest Diagnostics, Inc.
- Sciteck, Inc.
- Thermo Fisher Scientific, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Acm Global Laboratories
- Alfa Scientific Designs, Inc.
- Clinical Reference Laboratory, Inc.
- Express Diagnostics Int'l, Inc.
- Premier Brands of America, Inc.
- Quest Diagnostics, Inc.
- Sciteck, Inc.
- Thermo Fisher Scientific, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 173 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
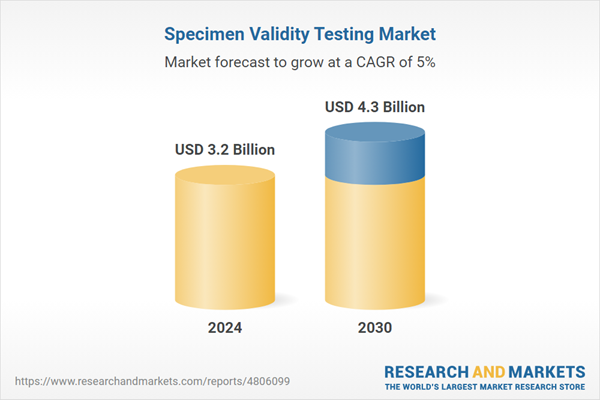
| Estimated Market Value ( USD | $ 3.2 Billion |
| Forecasted Market Value ( USD | $ 4.3 Billion |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |