Speak directly to the analyst to clarify any post sales queries you may have.
Unveiling the Critical Role and Strategic Significance of Laboratory Information Management Systems in Accelerating Scientific Innovation and Operational Excellence
Laboratory Information Management Systems have evolved from simple sample tracking tools into comprehensive platforms that underpin every aspect of modern research and testing operations. By integrating data capture, workflow automation, and compliance management into a unified ecosystem, these systems empower organizations to accelerate discovery, improve quality control, and maximize resource utilization. The increasing complexity of life sciences, clinical diagnostics, and environmental testing has driven institutions to adopt sophisticated informatics to manage high-throughput workflows, ensure traceability of critical data, and facilitate cross-functional collaboration.As laboratories confront mounting regulatory demands and escalating competitive pressures, the strategic significance of robust informatics systems cannot be overstated. Streamlined data management not only accelerates time to insight but also mitigates risks associated with manual processes, human error, and data silos. In aggregate, these efficiencies drive cost savings, enhance reproducibility, and support continuous innovation. Consequently, laboratory leaders are prioritizing investments in systems that offer modular scalability, configurable workflows, and seamless integration with emerging technologies such as artificial intelligence and the Internet of Things.
Exploring the Transformative Technological and Operational Shifts Redefining the Laboratory Information Management Systems Ecosystem and Driving Future Growth
The landscape of laboratory informatics is undergoing a profound transformation, driven by advancements in artificial intelligence, edge computing, and seamless data integration. Sophisticated analytics engines are now embedded within platforms, enabling predictive quality control, anomaly detection, and real-time decision support. This shift toward intelligence-driven operations is complemented by the proliferation of cloud-native solutions, which offer elasticity, global accessibility, and rapid deployment capabilities. As a result, laboratories can scale their computational resources on demand, integrate geographically dispersed data sources, and maintain operational continuity in dynamic environments.Additionally, the convergence of laboratory automation with informatics solutions is reshaping workflow paradigms. Robotic sample handlers, integrated IoT sensors, and automated reporting modules collaborate to minimize manual intervention, reduce turnaround times, and enforce standardized protocols. Regulatory landscapes have also evolved in tandem, requiring advanced compliance management functionalities to adhere to evolving quality standards and data integrity mandates. In light of these developments, laboratory leaders are compelled to adopt platforms that deliver both technological agility and rigorous governance frameworks.
Assessing the Far-Reaching Consequences of the United States’ 2025 Tariff Adjustments on Supply Chains, Costs, and Competitive Dynamics in Life Sciences
The United States’ tariff adjustments slated for 2025 have introduced a layer of complexity across laboratory supply chains, particularly affecting the import of specialized instruments, reagents, and consumables. Procurement teams are recalibrating supplier strategies to navigate higher duties on precision equipment and raw materials sourced from key manufacturing hubs. These cost pressures are prompting laboratories to explore alternative sourcing regions and renegotiate long-term contracts to maintain budgetary constraints.Moreover, the tariff regime has catalyzed a shift in pricing dynamics, with some vendors absorbing duties to preserve market share while others pass the full burden to end users. Consequently, laboratory managers must adopt proactive cost management approaches, leveraging consolidated procurement platforms and enhanced analytics to forecast expenditure impacts accurately. Against this backdrop, agility in adjusting to evolving trade policies and building diversified supplier networks has emerged as a critical capability for sustaining seamless operations and safeguarding research timelines.
Revealing Key Insights from Component, Deployment, Laboratory Size, and End User Segmentation Illustrating Market Dynamics and Opportunity Landscapes
Insights into component segmentation reveal that service offerings-encompassing consulting, implementation, and support maintenance-have become indispensable for laboratories seeking to optimize system performance and user adoption. Equally critical is the software segment, which spans compliance management, data management, document management, reporting analytics, and sample management. Each module addresses specialized needs, from ensuring regulatory adherence to enabling customizable dashboards that deliver actionable insights across complex datasets.Laboratory size also influences adoption patterns, with large-scale institutions prioritizing end-to-end integration and advanced analytics capabilities to support multi-site operations, while medium-sized entities balance feature richness with cost efficiencies, and smaller facilities emphasize user-friendly interfaces and rapid deployment schedules. Deployment mode preferences further underscore this diversity; cloud-based architectures-both private and public-are favored by organizations seeking scalability and remote collaboration, whereas on-premises implementations retain appeal among institutions prioritizing data sovereignty and legacy integration. Finally, end-user segmentation across academic and government research centers, biotechnology innovators, clinical research organizations, environmental testing labs, food and beverage quality control divisions, and pharmaceutical developers highlights distinct functional requirements and customization demands across sectors.
Uncovering Regional Dynamics and Strategic Imperatives Across the Americas, Europe Middle East Africa, and Asia-Pacific Laboratories and Research Hubs
In the Americas, robust research funding, a dynamic startup ecosystem, and regulatory harmonization have fostered widespread adoption of advanced informatics platforms. Major metropolitan clusters host leading institutions that continuously upgrade their systems to support high-throughput sequencing, precision medicine initiatives, and large-scale clinical trials. Meanwhile, Latin American laboratories are increasingly investing in modular solutions that can adapt to evolving research agendas and budgetary cycles.The Europe, Middle East, and Africa region presents a mosaic of environments, from established pharmaceutical research hubs in Western Europe to emerging clinical diagnostic networks in North Africa and the Gulf region. Laboratories here demonstrate a keen focus on compliance management and data security features, driven by stringent GDPR-like regulations and national health authority mandates. In the Asia-Pacific, rapid expansion of biotechnology clusters, government-sponsored innovation parks, and cross-border collaborations have fueled demand for cloud-enabled platforms that support multilingual interfaces, distributed data processing, and hybrid deployment models. Across each region, the interplay of regulatory frameworks, funding priorities, and technological maturity shapes distinct adoption curves.
Highlighting Leading Laboratory Information Management Providers, Their Strategic Positioning, Innovation Trajectories, and Competitive Advantages
The competitive landscape is defined by a blend of established enterprise software vendors and specialized laboratory informatics providers. These entities differentiate themselves through niche-focused modules, strategic partnerships, and robust service ecosystems. Leading firms emphasize end-to-end platform integration, forging alliances with instrument manufacturers and cloud infrastructure partners to deliver turnkey solutions that span sample management to regulatory reporting.Innovative start-ups and mid-tier players contrast this approach with laser-focused offerings, such as AI-driven analytics engines or mobile-enabled sample tracking tools designed for high-throughput laboratories. Their agility and rapid product iteration cycles enable them to address emerging use cases, from decentralized clinical trials to remote environmental monitoring. Across the spectrum, continuous investment in R&D, user experience design, and interoperability frameworks underpins competitive positioning and long-term growth trajectories.
Delivering Targeted Actionable Strategies for Industry Leaders to Navigate Regulatory, Technological, and Market Challenges in Laboratory Informatics
Industry leaders should prioritize investment in cloud-native architectures that facilitate real-time collaboration and modular scalability. By adopting hybrid deployment strategies, organizations can balance data sovereignty concerns with the agility benefits of public cloud environments. Additionally, integrating advanced artificial intelligence and machine learning capabilities will empower predictive quality control and resource optimization, driving operational efficiencies across R&D and production workflows.Furthermore, establishing strategic partnerships with instrument manufacturers, reagent suppliers, and regulatory consultants can streamline implementation timelines and enhance system interoperability. Emphasizing change management and user training programs will accelerate adoption and maximize return on investment. Finally, cultivating a data-centric culture-supported by standardized protocols and governance frameworks-will ensure data integrity, regulatory compliance, and better decision-making across multidisciplinary teams.
Detailing Rigorous Research Methodology Employed to Ensure Accuracy, Reliability, and Relevance in Laboratory Information Management Systems Insights
This research leverages a rigorous methodology combining primary interviews with laboratory managers, informatics specialists, and procurement executives alongside extensive secondary research from peer-reviewed journals, industry white papers, and regulatory documentation. Data triangulation processes ensure the validation of critical findings, while cross-referencing vendor announcements and product roadmaps provides current perspectives on technology trends.Segmentation analysis is underpinned by a comprehensive framework encompassing component, laboratory size, deployment mode, and end-user criteria. Regional insights draw on macroeconomic indicators, research funding patterns, and regulatory environment assessments. All data has undergone quality control checks, ensuring consistency and reliability in presenting actionable intelligence to decision-makers.
Synthesis of Critical Findings and Strategic Perspectives Underpinning the Future Evolution of Laboratory Information Management Systems
In summary, laboratory informatics platforms are at the forefront of modernizing research and testing workflows, driving efficiency, compliance, and innovation. The convergence of cloud-based architectures, AI-driven analytics, and integrated automation is reshaping the capabilities expected from these systems. Regional variations and tariff-driven supply chain shifts underscore the importance of flexible deployment strategies and diversified sourcing models.By understanding component, size, deployment, and end-user segmentation, industry stakeholders can identify high-impact opportunities and tailor their technology roadmaps accordingly. The competitive environment rewards both comprehensive suites and specialized solutions, with success hinging on agility, strategic partnerships, and a relentless focus on data integrity. As laboratories navigate evolving regulatory, technological, and market landscapes, informed decision-making and proactive strategy execution will determine leadership in the dynamic world of laboratory informatics.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Laboratory Information Management Systems Market
Companies Mentioned
The key companies profiled in this Laboratory Information Management Systems market report include:- Abbott Laboratories
- ABI Health
- Agile Frameworks, LLC
- Apex Healthware
- Autoscribe Informatics
- Benchling
- Biodata Inc
- Computing Solutions, Inc.
- Eusoft
- Illumina, Inc.
- International Business Machines Corporation
- LabLynx, Inc.
- LabVantage Solutions, Inc.
- LabWare Holdings
- LABWORKS, LLC
- Oracle Corporation
- PerkinElmer Inc
- QBench Inc.
- SciNote LLC
- Semaphore Solutions
- Soft Computer Consultants, Inc.
- STARLIMS Corporation
- Sunquest Laboratory by Clinisys
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | January 2026 |
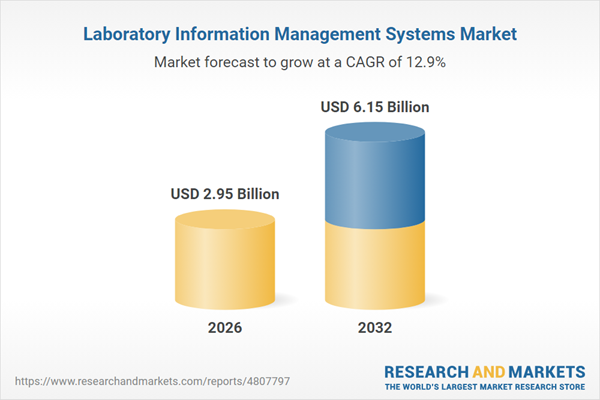
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 2.95 Billion |
| Forecasted Market Value ( USD | $ 6.15 Billion |
| Compound Annual Growth Rate | 12.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |