Global Creatinine Assay Kits Market - Key Trends and Drivers Summarized
Kidney Check: Why Are Creatinine Assay Kits Crucial in Medical Diagnostics?
Creatinine assay kits are essential tools in clinical settings, providing quick and accurate measurements of creatinine levels in blood and urine samples. Creatinine, a waste product generated from muscle metabolism, is a key indicator of kidney function. These kits utilize various methodologies, including Jaffe's kinetic method and enzymatic assays, to evaluate creatinine concentration. The results help healthcare providers assess the glomerular filtration rate (GFR), a critical parameter in diagnosing and monitoring kidney diseases. As kidney function declines, creatinine levels rise, signaling potential renal impairment or failure. Thus, creatinine assays play a pivotal role in early detection and ongoing management of kidney diseases, ensuring timely intervention and treatment customization.How Have Advancements in Creatinine Assay Technology Enhanced Patient Care?
The field of creatinine testing has seen significant technological advancements that have enhanced the accuracy, efficiency, and convenience of these tests. Modern creatinine assay kits are designed for high compatibility with automated analyzers, allowing for seamless integration into routine laboratory workflows. These advancements include improved reagent formulations that reduce interference from other substances in the sample, ensuring more reliable results. Additionally, the development of point-of-care creatinine testing devices has revolutionized patient management, especially in critical care and emergency settings, by providing rapid results that support immediate clinical decisions. Such innovations not only optimize patient care but also significantly decrease the turnaround time compared to traditional laboratory methods.What Challenges Influence the Use of Creatinine Assay Kits?
Despite their benefits, the deployment of creatinine assay kits faces several challenges. One major issue is the variability in assay results due to differences in test design and sensitivity among different manufacturers. This can lead to inconsistencies in creatinine measurements, affecting clinical decisions related to kidney health management. Furthermore, the presence of certain chemicals or drugs in patients' samples can interfere with the accuracy of creatinine assays, leading to either falsely elevated or reduced levels. Addressing these challenges requires ongoing research and standardization efforts to ensure that creatinine assays remain reliable and effective across diverse clinical environments and populations.What Are the Major Drivers Propelling the Creatinine Assay Kits Market Forward?
The growth in the creatinine assay kits market is driven by several factors, reflecting an increased focus on preventive healthcare and chronic disease management. The rising prevalence of conditions such as hypertension and diabetes, which are known risk factors for chronic kidney disease (CKD), has led to greater use of creatinine assays in routine health screenings. Advances in healthcare infrastructure, particularly in developing countries, have expanded access to these diagnostic tests, further propelling market growth. Additionally, the aging global population, which is more susceptible to renal function decline, underscores the need for regular kidney function monitoring. Technological advancements that improve the accuracy and user-friendliness of these kits also contribute to their increased adoption. As health systems worldwide emphasize early detection and management of kidney disease to improve patient outcomes and reduce healthcare costs, the demand for creatinine assay kits is expected to continue rising.Report Scope
The report analyzes the Creatinine Assay Kits market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below.- Segments: Type (Jaffe's Kinetic Test Kits, Creatinine-PAP Test Kits, ELISA Test Kits); Sample (Urine, Blood / Serum, Other Samples).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Jaffe's Kinetic Test Kits segment, which is expected to reach US$102.1 Million by 2030 with a CAGR of 2.1%. The Creatinine-PAP Test Kits segment is also set to grow at 2.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $52.9 Million in 2024, and China, forecasted to grow at an impressive 2.1% CAGR to reach $36.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Creatinine Assay Kits Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Creatinine Assay Kits Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Creatinine Assay Kits Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Abcam PLC, Arbor Assays Inc., BioAssay Systems, Biovision Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 46 companies featured in this Creatinine Assay Kits market report include:
- Abbott Laboratories
- Abcam PLC
- Arbor Assays Inc.
- BioAssay Systems
- Biovision Inc.
- Cayman Chemical Company
- Cell Biolabs, Inc.
- Crystal Chem, Inc.
- Enzo Life Sciences, Inc.
- Genway Biotech, Inc.
- Merck KgaA
- Quidel Corporation
- Thermo Fisher Scientific, Inc.
- Tulip Diagnostics (P) Ltd.
- Wako Pure Chemical Industries Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Abcam PLC
- Arbor Assays Inc.
- BioAssay Systems
- Biovision Inc.
- Cayman Chemical Company
- Cell Biolabs, Inc.
- Crystal Chem, Inc.
- Enzo Life Sciences, Inc.
- Genway Biotech, Inc.
- Merck KgaA
- Quidel Corporation
- Thermo Fisher Scientific, Inc.
- Tulip Diagnostics (P) Ltd.
- Wako Pure Chemical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
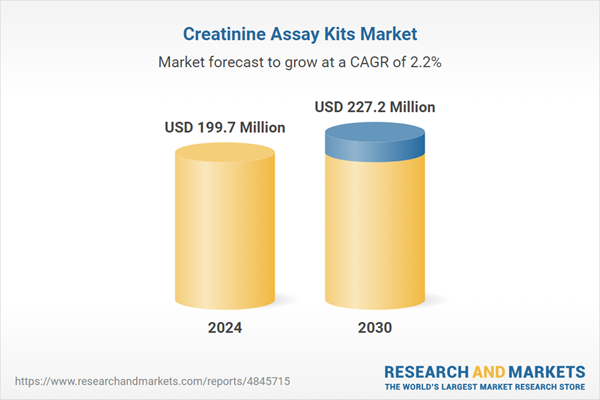
| Estimated Market Value ( USD | $ 199.7 Million |
| Forecasted Market Value ( USD | $ 227.2 Million |
| Compound Annual Growth Rate | 2.2% |
| Regions Covered | Global |