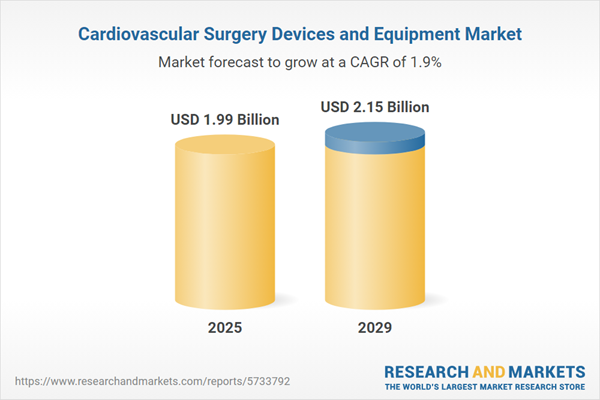
The cardiovascular surgery devices and equipment market size has grown strongly in recent years. It will grow from $1.89 billion in 2024 to $1.99 billion in 2025 at a compound annual growth rate (CAGR) of 5.3%. The growth in the historic period can be attributed to prevalence of cardiovascular diseases, technological advancements, aging population, chronic disease management.
The cardiovascular surgery devices and equipment market size is expected to see marginal growth in the next few years. It will grow to $2.15 billion in 2029 at a compound annual growth rate (CAGR) of 1.9%. The growth in the forecast period can be attributed to minimally invasive surgery, advanced imaging, personalized medicine, telemedicine and teleconsultations. Major trends in the forecast period include hybrid operating rooms, transcatheter procedures, artificial intelligence (ai) in surgery, remote surgical training.
The expanding elderly demographic combined with the increasing prevalence of fatalities attributed to cardiovascular ailments serves as a driving force in the cardiovascular surgical devices market. As per the American Heart Association (AHA), approximately 43.7 million individuals over 60 years old in the United States are affected by cardiovascular conditions. AHA reports an estimated 17.3 million deaths annually from cardiac diseases, which are anticipated to exceed 23.6 million worldwide by 2030. Consequently, a greater number of surgical procedures are being conducted, thereby spurring market growth.
The projected growth in the cardiovascular surgery devices and equipment market is attributed to the escalating number of individuals affected by obesity. Obesity, characterized by excessive body fat accumulation, is linked to various severe health complications, including cardiovascular disease, diabetes, and specific forms of cancer. Utilizing cardiovascular surgery devices and equipment in the treatment of obese patients can significantly enhance surgical outcomes. According to the Trust for America's Health's State of Obesity 2022 report, 40% of American adults are grappling with obesity, and this rate is steadily climbing both nationwide and across different demographic groups. Specifically, 19 states have seen obesity rates surpassing 35%, a notable increase from the figures reported in 2021. This surge in obesity cases underpins the growth of the cardiovascular surgery devices and equipment market.
Major companies are investing in businesses operating in the cardiovascular surgery devices and equipment market, focusing on developing innovative solutions such as CardioVisio for Atrial Fibrillation (AFib) to improve early diagnosis, enhance patient outcomes, and streamline treatment pathways. CardioVisio for Atrial Fibrillation (AFib) is an advanced diagnostic and monitoring tool designed to detect and manage AFib, a common form of irregular heart rhythm. For instance, in August 2023, GE HealthCare, a U.S.-based medical technology company, launched CardioVisio for Atrial Fibrillation (AFib), a digital clinical decision support tool aimed at enhancing precision care for AFib patients. Introduced at the ESC Congress 2023, this innovative solution integrates longitudinal patient data from multiple sources, offering clinicians a comprehensive view of heart health. By providing evidence-based recommendations aligned with AFib guidelines, CardioVisio helps streamline clinical workflows and improves adherence to best practices.
Major players in the Cardiovascular Surgery Devices And Equipment market are actively investing in companies within the sector to solidify their market presence. These companies allocate significant investments in research and development to create pioneering products that enhance patient outcomes and shorten recovery times. For example, in August 2023, Capstan Medical, a US-based medical device firm specializing in robotic-assisted heart valve surgery technology, secured $31 million in Series B funding. This funding infusion is intended to support the development of robotic transcatheter heart valve replacements, focusing on the integration of robotics into minimally invasive heart valve procedures. Capstan Medical's technology is engineered to introduce robotic precision into the placement and deployment of valve implants within a functioning heart.
The U.S. Food and Drug Administration's (FDA) Center for Devices and Radiological Health (CDRH) has unveiled its priority list for medical device guidance documents. This action is set to support companies by fostering innovation and safeguarding them against the rigorous implications of product disapproval. As a result, a more defined and transparent approval process is expected to stimulate growth in the cardiovascular surgical devices market in the upcoming period.
Cardiovascular surgery devices and equipment are crucial tools used in procedures focused on repairing structural defects in the cardiovascular system and addressing issues with valves and vessels.
The primary categories of cardiovascular surgery devices and equipment encompass beating heart surgery systems, cardiopulmonary bypass equipment, cardiac ablation devices, and perfusion disposables. Cardiopulmonary bypass equipment, particularly utilized in open-heart surgeries, is operated with precision. These tools find various applications in treating congenital heart defects, cardiac arrhythmia, coronary heart disease, congestive heart failure, among other medical needs. They are extensively employed in hospitals, diagnostic laboratories, research laboratories, as well as home and ambulatory care settings.
The cardiovascular surgery devices and equipment market research report is one of a series of new reports that provides cardiovascular surgery devices and equipment market statistics, including cardiovascular surgery devices and equipment industry global market size, regional shares, competitors with a cardiovascular surgery devices and equipment market share, detailed cardiovascular surgery devices and equipment market segments, market trends and opportunities, and any further data you may need to thrive in the cardiovascular surgery devices and equipment industry. This cardiovascular surgery devices and equipment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Major companies operating in the cardiovascular surgery devices and equipment market include Abbott Laboratories, Boston Scientific Corporation, Cook Medical, Abiomed Inc., Angiodynamics, Transmedics, Terumo cardiovascular systems corporation, Edwards Life Science Corporation, Medtronic PLC, Cardinal Health, Cyberheart Incorporated, Medwaves Incorporated, Thoratec Corporation, Cordis Corporation, Getinge Group, Atrion Corporation, Sorin S.P.A., Biosensors International, Bioventrix Inc., Maquet GmbH & Co. KG, EndoPhotonix Inc., Krdium Inc., SEMMT Inc., Amaranth Medical, 3M Health Care, Abaxis Inc., Atlas Genetics, Becton Dickinson and Company, BioFire Diagnostics, bioMérieux, Bio-Rad Laboratories, Cepheid, Chembio Diagnostic Systems, EKF Diagnostics, GenMark Diagnostics, Hologic, LumiraDx, Mesa Biotech (a subsidiary of Thermo Fisher Scientific), Nova Biomedical, NOWDiagnostics, Opko Health, OraSure Technologies, QIAGEN, Roche Diagnostics, Sekisui Diagnostics, Siemens Healthineers, Sysmex Corporation, Thermo Fisher Scientific, Trividia Health, Trivitron Healthcare, Werfen S.A.
North America was the largest region in the global cardiovascular surgery devices and equipment market in 2024. Western Europe was the second-largest region in the global cardiovascular surgery devices and equipment market. The regions covered in the cardiovascular surgery devices and equipment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa. The countries covered in the cardiovascular surgery devices and equipment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
The cardiovascular surgery devices and equipment market consist of sales of perfusion disposables, beating heart surgery systems, cardiopulmonary bypass equipment, and cardiac ablation devices that are used for cardiovascular surgeries. Values in this market are factory gate values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Executive Summary
Cardiovascular Surgery Devices and Equipment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on cardiovascular surgery devices and equipment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 50 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for cardiovascular surgery devices and equipment ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The cardiovascular surgery devices and equipment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product Type: Beating heart surgery systems; Cardiopulmonary Bypass Equipment; Cardiac Ablation Devices; Perfusion Disposables2) By Application: Congenital Heart Defects; Cardiac Arrhythmia; Coronary Heart Disease; Congestive Heart Failure; Other Applications
3) By End User: Home and Ambulatory care; Hospitals; Diagnostic laboratories; Research Laboratories
Subsegments:
1) By Beating Heart Surgery Systems: Stabilization Systems; Tissue Stabilizers; Coronary Artery Bypass Surgery Systems; Other Beating Heart Surgery Devices2) By Cardiopulmonary Bypass Equipment: Heart-Lung Machines; Oxygenators; Cannulas; Tubing Packs; Cardioplegia Delivery Systems; Other Cardiopulmonary Bypass Equipment
3) By Cardiac Ablation Devices: Radiofrequency Ablation Devices; Cryoablation Devices; Laser Ablation Devices; Microwave Ablation Devices; Other Cardiac Ablation Devices
4) By Perfusion Disposables: Blood Oxygenators; Perfusion Cannulas; Tubing Sets; Filters; Blood Reservoirs; Other Perfusion Disposables
Key Companies Mentioned: Abbott Laboratories; Boston Scientific Corporation; Cook Medical; Abiomed Inc.; Angiodynamics
Countries: Australia; China; India; Indonesia; Japan; South Korea; Bangladesh; Thailand; Vietnam; Malaysia; Singapore; Philippines; Hong Kong; New Zealand; USA; Canada; Mexico; Brazil; Chile; Argentina; Colombia; Peru; France; Germany; UK; Austria; Belgium; Denmark; Finland; Ireland; Italy; Netherlands; Norway; Portugal; Spain; Sweden; Switzerland; Russia; Czech Republic; Poland; Romania; Ukraine; Saudi Arabia; Israel; Iran; Turkey; UAE; Egypt; Nigeria; South Africa
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Cardiovascular Surgery Devices and Equipment market report include:- Abbott Laboratories
- Boston Scientific Corporation
- Cook Medical
- Abiomed Inc.
- Angiodynamics
- Transmedics
- Terumo cardiovascular systems corporation
- Edwards Life Science Corporation
- Medtronic plc
- Cardinal Health
- Cyberheart Incorporated
- Medwaves Incorporated
- Thoratec Corporation
- Cordis Corporation
- Getinge Group
- Atrion Corporation
- Sorin S.P.A.
- Biosensors International
- Bioventrix Inc.
- Maquet GmbH & Co. KG
- EndoPhotonix Inc.
- Krdium Inc.
- SEMMT Inc.
- Amaranth Medical
- 3M Health Care
- Abaxis Inc.
- Atlas Genetics
- Becton Dickinson and Company
- BioFire Diagnostics
- bioMérieux
- Bio-Rad Laboratories
- Cepheid
- Chembio Diagnostic Systems
- EKF Diagnostics
- GenMark Diagnostics
- Hologic
- LumiraDx
- Mesa Biotech (a subsidiary of Thermo Fisher Scientific)
- Nova Biomedical
- NOWDiagnostics
- Opko Health
- OraSure Technologies
- QIAGEN
- Roche Diagnostics
- Sekisui Diagnostics
- Siemens Healthineers
- Sysmex Corporation
- Thermo Fisher Scientific
- Trividia Health
- Trivitron Healthcare
- Werfen S.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.99 Billion |
| Forecasted Market Value ( USD | $ 2.15 Billion |
| Compound Annual Growth Rate | 1.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 51 |