Speak directly to the analyst to clarify any post sales queries you may have.
The hematology analyzers and reagents market is a cornerstone of modern clinical diagnostics, providing laboratories and healthcare systems with the solutions needed to refine workflows, support effective patient management, and strengthen evidence-based decision-making. It consistently drives advancements that shape healthcare delivery and operational processes.
Market Snapshot: Hematology Analyzers & Reagents Market
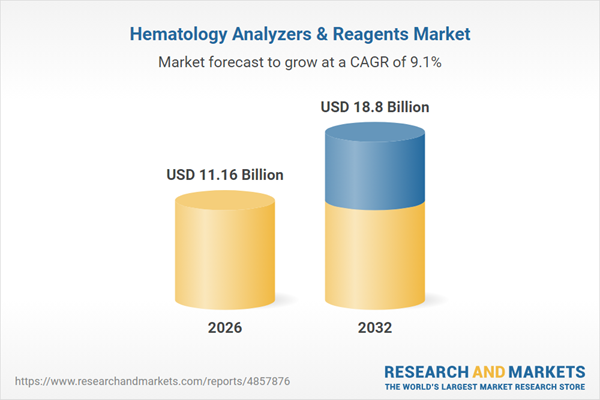
The Hematology Analyzers & Reagents Market is experiencing robust growth, having expanded from USD 10.21 billion in 2025 to USD 11.16 billion in 2026. Projections indicate the market will reach USD 18.80 billion by 2032, supported by a CAGR of 9.10%. This upward trajectory is underpinned by investments in advanced analytical innovation, improvements in reagent stability, and heightened focus on supply chain resilience among key stakeholders. Increasing demand spans from centralized reference labs to decentralized clinical settings and resource-limited environments. These market dynamics are prompting manufacturers and service providers to prioritize flexible, easy-to-integrate diagnostic solutions tailored to diverse end-user requirements.
Scope & Segmentation
- Reagent Types: Control reagents ensure consistent quality with reliable batch performance. Diluent reagents manage instrument fluidic operations and impact lab efficiency. Reticulocyte reagents facilitate specialized analyses vital for tracking disorders like anemia and monitoring bone marrow activity.
- Applications: The technologies are widely utilized across blood disorder management, pharmaceutical R&D, infectious disease monitoring, and health screening programs. Each application comes with distinct needs in throughput, result specificity, and reporting standards.
- End Users: Blood banks, clinical labs, hospitals, and research institutions each implement distinct procurement strategies, validation protocols, and support requirements based on scale and regulatory context.
- Distribution Channels: Direct sales allow providers to offer tailored technical support and robust integration, whereas distributor models facilitate wider geographic coverage and help navigate regulatory complexities—particularly in regions requiring specific logistical solutions.
- Regional Dynamics: The Americas prioritize high-throughput automation and seamless integration within hospital systems. The Europe, Middle East & Africa region emphasizes traceability, regulatory compliance, and flexible logistics. Asia-Pacific markets are investing in new laboratory infrastructure, demanding durable, low-maintenance analyzers and reagents.
- Key Technologies: Innovations include digitized signal processing, microfluidic handling, enhanced cell phenotyping, and stabilized chemistries that collectively raise analytical performance standards and support uninterrupted operations for labs globally.
Key Takeaways for Senior Decision-Makers
- Modern hematology analyzers deliver precise clinical results while increasing laboratory productivity, which supports more integrated and reliable diagnostic processes.
- Effective management of reagent life cycles, including supplier reliability and consistent quality, is vital for ensuring operational continuity and meeting regulatory demands.
- Adoption of digital technologies introduces proactive quality controls, streamlining compliance procedures and addressing increased expectations around laboratory efficiency and reporting.
- Companies offering interoperable systems, broad reagent choices, and flexible support have opportunities to meet specialized regional and institutional requirements more effectively.
- Procurement strategies are evolving to focus on supplier performance after purchase, regulatory readiness, and the ability to manage risks throughout complex healthcare settings.
Tariff Impact: Strategic Shifts in Supply Chains
Fluctuating tariffs are prompting significant adjustments in sourcing and supply chain strategies throughout the hematology diagnostics sector. Healthcare organizations and suppliers are re-evaluating supplier contracts, logistics pathways, and inventory strategies to address changing cost structures and uncertainty. This climate is encouraging nearshoring and regional manufacturing collaborations to enhance supply chain agility. As a result, decision-makers are prioritizing domestic production capabilities and robust contract structures during supplier selection, directly influencing procurement routes and long-term operational planning.
Methodology & Data Sources
This report is based on a multi-source approach that includes primary interviews with procurement leaders, laboratory directors, and technical experts. In addition, technical documentation, regulatory sources, and real-world performance reviews are analyzed to ensure thorough and validated market intelligence.
Why This Report Matters
- Presents actionable insights into market trends, technological innovation, and regulatory transformation, empowering healthcare leaders and suppliers to make informed procurement and strategy choices.
- Explores the impact of emerging technologies and operational requirements on supplier relationships and industry competition.
- Supports decision-making by evaluating partner reliability, digital system synchronization, and consistent supply—elements critical for resilient laboratory performance.
Conclusion
Tapping into progress in hematology analyzers and reagents is essential to maintain the quality and efficiency of clinical diagnostics. Emphasis on chemistry reliability, system compatibility, and resilient supply infrastructure will underpin future success in healthcare operations.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Hematology Analyzers & Reagents Market
Companies Mentioned
The key companies profiled in this Hematology Analyzers & Reagents market report include:- Abbott Laboratories
- ACON Laboratories, Inc.
- Beckman Coulter, Inc. by Danaher Corporation
- BIOBASE Group
- Bioevopeak Co., Ltd.
- Biogenix Inc. Pvt. Ltd.
- Biosystems S.A. by Ginper S.L.
- Boule Diagnostics AB
- Cellavision AB
- Chengdu Seamaty Technology Co., Ltd.
- CPC Diagnostics Pvt. Ltd.
- Diatron Medical Instruments Limited
- Drucker Diagnostics, LLC
- EDAN Instruments, Inc.
- EKF Diagnostics Holdings Plc
- ERBA Diagnostics Mannheim GmbH
- F. Hoffmann-La Roche Ltd.
- Genrui Biotech Co., Ltd.
- Getein Biotech, Inc.
- HORIBA, Ltd
- Labnics Equipment Ltd.
- Labomed Inc.
- Linear Chemicals S.L.U.
- Medtronic PLC
- Mindray Bio-Medical Electronics Co., Ltd.
- Nihon Kohden Corporation
- Nova Biomedical Corporation
- PZ Cormay S.A.
- Siemens AG
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- Trivitron Healthcare
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 11.16 Billion |
| Forecasted Market Value ( USD | $ 18.8 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 33 |