The COVID-19 pandemic led to interruptions in routine patient care. Many healthcare organizations canceled routine clinic visits and elective surgeries to increase and maintain the availability of intensive care and inpatient care beds and have reorganized medical and nursing staff. This had particularly interrupted care for patients with chronic rhinitis. Also, several adjacent infections were widely reported in COVID-19 infected patients, like fungal sinusitis. For instance, as per the article published in February 2022 in JFMPC, researchers reported that many patients presented with clinical signs and symptoms of fungal infection with a history of COVID-19. This occurred due to the multiple secondary fungal infections because of immune dysregulation, widespread use of steroids, high glycemic index, prolonged hospital stay in intensive care units, and increased ferritin levels. However, with the decreasing number of cases, the long-term impact of the SARS-CoV-2 virus on humans, and large-scale vaccination programs worldwide, the studied market is expected to regain its full growth potential over the forecast period of the study.
The increasing popularity and demand for minimally invasive surgeries owing to their benefits such as smaller incisions, less scarring, high accuracy and decreased risk of complications, shorter hospital stays, and others, coupled with the high burden of sinusitis around the world are expected to be significant growth factors that are fueling growth in the sinus dilation devices market. Further, with the growing number of surgeries and increasing demand for minimally invasive surgical procedures, the demand for sinus dilation products is expected to increase, which will boost growth in the studied market over the forecast period of the study. For instance, in February 2021, Intersect ENT, Inc. launched its new Straight Delivery System packaged with the company’s PROPEL Mini (mometasone furoate) Sinus Implant. The combined packaging of the SDS with PROPEL Mini received premarket approval from the FDA. Hence, with the rising number of sinus-related surgeries, the demand for sinus dilation products is expected to increase, which is expected to fuel growth in the studied market.
Thus, due to the rise sinus related issues and the surge in sinus dilation product launches, the studied market is anticipated to grow over the forecast period. However, factors such as risks, complications associated with sinus surgeries, and lack of skilled healthcare professionals are expected to restrain the growth of the sinus dilation devices market during the forecast period.
Sinus Dilation Devices Market Trends
Balloon Sinus Dilation Device is Expected to Hold Significant Market Share in the Product Segment During Forecast Period
The balloon sinus dilation device is used as a minimally invasive technique to treat chronic or recurrent sinusitis or sinus infections when medical therapy has not provided adequate relief. During this procedure, surgeons use a small balloon placed through the nose to dilate the sinus openings. Factors such as an increase in the adoption of balloon sinus dilation procedures, a rise in sinus infection, and technological advancements are expected to drive segment growth over the forecast period. There are several balloon sinus dilation devices available in the market, such as the NuVent balloon sinus dilation system from Medtronic, Audion ET dilation system from Stryker Corporation, and Mesire Sinus Balloon Catheter from Meril Life Sciences, among many others.According to a review study published in December 2021 on balloon sinuplasty in PubMed, over the past ten years, the use of balloon sinuplasty for the treatment of chronic rhinosinusitis (CRS) that is resistant to conventional medical therapy significantly increased. The number of patients choosing to undergo this procedure is increasing because of its decreased risk of complications, quicker recovery time, and minimally invasive nature. Hence, this segment is expected to have a significant market share during the forecast period of the study.
Moreover, the ongoing research and development activities on further improving the balloon sinus dilation devices, coupled with the technological innovations and launch of new devices, are further expected to boost the demand for balloon sinus dilation devices during the forecast period. For instance, in June 2021, Intersect ENT launched an advanced VenSure balloon sinus dilation system along with a Cube 4D navigation system in the United States. The VenSure Balloon and Cube 4D Navigation Systems are used in procedures that are designed to improve debilitating chronic rhinosinusitis (CRS) symptoms. Therefore, such innovations and technologically advanced product launches in balloon sinus dilation devices are likely to drive segment growth over the forecast period.
Thus, due to the rise in technological advancements in balloon sinus dilation procedures and the surge in balloon sinus dilation device product launches, the studied segment is anticipated to grow over the forecast period.
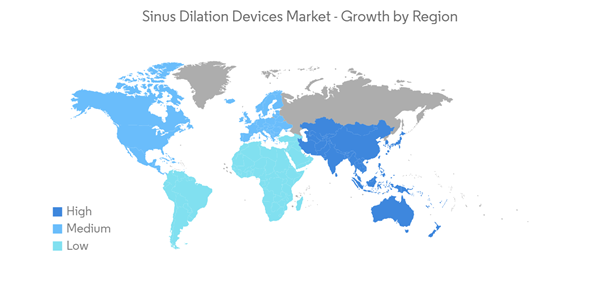
North America is Expected to Hold a Significant Share in the Market Over the Forecast Period
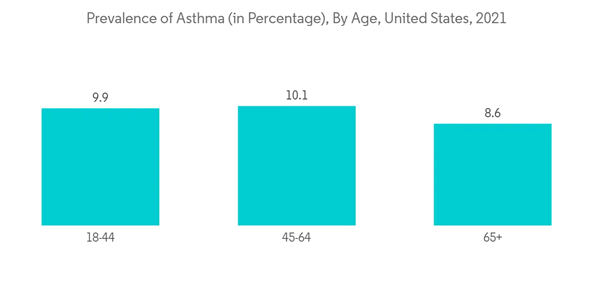
North American region is expected to occupy a significant share in the sinus dilation devices market due to the factors such as the presence of robust healthcare infrastructure, the high burden of sinusitis and related diseases, the launch of new products, and the increased burden of risks factors causing sinusitis in the region such as smoking, chronic cold, allergies, asthma, head injury, and other factors. Smoking can contribute to sinus infections because the toxins in cigarettes can damage the cilia in the nasal cavity. As per the article updated in June 2022 in SAGE journal, large epidemiologic studies suggested that a history of tobacco use may be associated with an increased risk of developing chronic rhinosinusitis (CRS). Moreover, according to the Canadian Tobacco and Nicotine Survey (CTNS): Summary of Results for 2021, approximately 13.3% (3.3 million) of Canadian people aged 25 and over reported smoking cigarettes, and men (13% or 1.7 million) and women (12% or 1.6 million) both reported a similar level of current smoking prevalence. Hence, the increase in tobacco utilization and smoking leads to the burden of sinusitis, driving demand for sinus dilation devices over the forecast period.Similarly, severe sinus infections lead to complicated asthma cases. As per the August 2022 article update in PubMed, chronic sinusitis is known to aggravate asthma and can potentially result in meningitis and brain abscess formation, increasing morbidity and mortality. Additionally, in the January 2022 update of the CDC, about 28.9 million adults in the United States are diagnosed with sinusitis, which is about 11.6% of the country's total adult population. Further, as per the same source, about 2.7 million visits are made to physician offices with chronic sinusitis as the primary diagnosis in the country. Thus, the high burden of sinusitis is expected to be the major driving factor for the growth of the sinus dilation devices market in the studied region. Furthermore, in July 2022, Zsquare, a developer of high-performance, single-use endoscopes, received FDA 510K clearance to market its first product, the Zsquare ENT-Flex Rhinolaryngoscope. Zsquare plans a pilot launch in leading United States hospitals and physician practice offices by Q4 2022. Hence, such product launches in the studied region raise product availability and demand, thereby driving the market growth.
Thus, North America is anticipated to grow over the forecast period due to the rise in the burden of sinus-related diseases and the surge in device product launches.
Sinus Dilation Devices Industry Overview
The sinus dilation devices market is moderately competitive. It comprises several significant players such as Smith and Nephew, Johnson & Johnson Inc., Medtronic, Meril Life Sciences Private Limited, and Olympus Corporation, among many others. The market is expected to grow more competitive with the launch of new devices, mergers and acquisitions, partnerships, and other business expansion activities by the key market players.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Johnson & Johnson
- Medtronic Plc
- Meril Life Sciences Private Limited
- Olympus Corporation
- SinuSys Corporation
- Smith & Nephew
- Stryker Corporation
- TE Connectivity (Creganna Medical)
- Dalent Medical
- InnAccel
- Accurate Surgical & Scientific Instruments
- KARL STORZ