The human papillomavirus (HPV) vaccine market is expected to register a CAGR of nearly 7.5% during the forecast period.
As the pandemic hit, the screening and diagnosis of non-emergency diseases were postponed worldwide, which has impacted the studied market. As per a research study published in March 2021 in PubMed, 74% of the total respondent laboratories globally experienced a supply shortage for HPV tests for cervical cancer screening. Therefore, there was a reduction in cervical cancer and other related cancer screening, ultimately reducing the demand for the HPV vaccine during the pandemic. Furthermore, a research study published in January 2023 in Vaccines indicated that a 75% reduction in HPV vaccination coverage was observed in the United States during the initial phase of the pandemic as compared to the previous year. Hence, owing to the factors mentioned above, the COVID-19 pandemic significantly impacted the market studied. However, with the COVID-19 cases in control, the market regained its pre-pandemic nature in terms of demand for HPV vaccines. Furthermore, due to the increasing initiatives by government & private organizations for early screening & vaccination and approval of new HPV vaccines, the market is believed to witness significant growth over the forecast period.
The rising prevalence of HPV-related diseases such as cervical cancer, anal cancer, and genital warts is expected to boost the demand for HPV vaccines significantly. For instance, as per the India, HPV and Related Cancers, 2023 report, cervical cancer ranks as the 2nd most frequent cancer among women in India and the 2nd most frequent cancer among women between 15 and 44. As per the same source, 123,907 women are diagnosed with cervical cancer in India each year. Therefore, the high burden of cervical cancer is expected to boost the demand for HPV vaccine during the forecast period.
The initiatives by the government to fund the HPV vaccines and rising screening and immunization programs are expected to boost the market growth. For instance, according to an article published in January 2023 in Vaccines Journal, the HPV vaccine is fully funded for children aged 11-14 years old and for 15-26-year-old females in Switzerland. Also, in March 2022, in Greece, a gender-neutral HPV vaccination program was launched.
Furthermore, in January 2021, the United States Department of Health and Human Services launched the "HPV VAX NOW" campaign to increase HPV vaccination rates among young adults aged 18-26 years. Also, in November 2021, Saudi Arabia launched a human papillomavirus infection program for young girls aged 9-13 years to prevent them from developing cervical cancer. This is expected to increase the development of HPV-cervical cancer vaccines hence bolstering the market growth.
Therefore, the factors such as the high burden of cervical cancer and rising screening and immunization programs, and launches of vaccines, the studied market is expected to witness growth during the forecast period. However, stringent regulation is likely to hinder market growth over the forecast period.
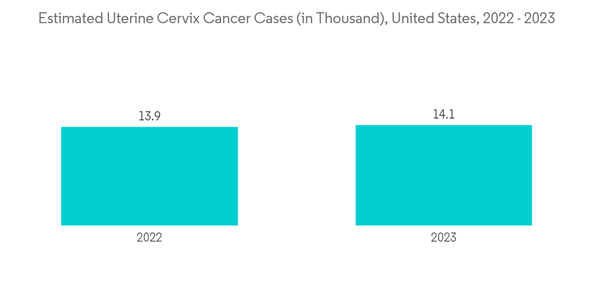
According to the American Cancer Society (ACS) 2023 report, an estimated 13,960 new cases of cervical cancer are predicted to be diagnosed in the United States in 2023. Moreover, as per Japan Human Papillomavirus, Fact Sheet 2023, every year, 12,785 women are diagnosed with cervical cancer, with an incidence of HPV-related cervical cancer of 19.8 in 2023. Therefore, the high burden of cervical cancer will boost the demand for developing innovative vaccines, propelling market growth.
Furthermore, the rising screening and awareness initiatives increase the diagnoses, further expected to boost the market growth. For instance, in February 2022, the Department of Health and Social Care (DHSC), with the support of the National Health Service (NHS), launched a new national cervical screening campaign to boost the number of eligible candidates to attend their cervical screening in England. The increased screening is projected to boost the demand for the HPV vaccine to provide protection against the high-risk HPV types associated with cervical cancer, thereby propelling the segment's growth.
The rising research and development to launch innovative vaccines is projected to boost the market growth. For instance, in September 2022, the Government of India launched the Quadrivalent Human Papillomavirus vaccine (qHPV) against cervical cancer in India, which was developed by the Serum Institute of India (SII) and the Department of Biotechnology (DBT). As per the SII, the vaccine would cost around USD 2.5 - USD 5 (INR 200-400) per dose, which is significantly lower compared to other parts of the world.
Thus, owing to the increasing cases of cervical cancer, rising cervical cancer screening, and growing vaccine launches, the segment is expected to witness high growth over the forecast period.
According to the American Cancer Society 2022 report, 9,440 new anal cancer were diagnosed in the United States in 2022. Moreover, per the Canadian Cancer Society, 1,450 Canadian women were diagnosed with cervical cancer in 2022. Therefore, the high burden of such cancers is expected to boost the growth of the studied market in the region during the forecast period.
In addition, according to an article published in February 2023 in the Human Vaccine and Immunotherapeutic Journal, HPV vaccination in the United States can start at age nine and last until age 26. Also, approximately 77% of 13 to 17-year-old teenagers have received one or more doses of the HPV vaccine, and nearly 62% have finished the entire series. The high coverage for the HPV vaccine and early screening initiatives in the region boost the demand for HPV vaccines during the forecast period.
Furthermore, the strategic initiatives by market players, such as launches and approvals, partnerships, and collaborations, also accelerate the market growth. For instance, in April 2022, Merck Canada received Health Canada's approval for an expanded indication of GARDASIL 9 ((Human Papillomavirus 9-valent Vaccine, Recombinant) in individuals 9 through 45 years of age to prevent infection caused by the Human Papillomavirus (HPV) types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Therefore, factors such as the growing cancer burden and vaccine launches and approvals are expected to boost the market growth in North America during the forecast period.
This product will be delivered within 2 business days.
As the pandemic hit, the screening and diagnosis of non-emergency diseases were postponed worldwide, which has impacted the studied market. As per a research study published in March 2021 in PubMed, 74% of the total respondent laboratories globally experienced a supply shortage for HPV tests for cervical cancer screening. Therefore, there was a reduction in cervical cancer and other related cancer screening, ultimately reducing the demand for the HPV vaccine during the pandemic. Furthermore, a research study published in January 2023 in Vaccines indicated that a 75% reduction in HPV vaccination coverage was observed in the United States during the initial phase of the pandemic as compared to the previous year. Hence, owing to the factors mentioned above, the COVID-19 pandemic significantly impacted the market studied. However, with the COVID-19 cases in control, the market regained its pre-pandemic nature in terms of demand for HPV vaccines. Furthermore, due to the increasing initiatives by government & private organizations for early screening & vaccination and approval of new HPV vaccines, the market is believed to witness significant growth over the forecast period.
The rising prevalence of HPV-related diseases such as cervical cancer, anal cancer, and genital warts is expected to boost the demand for HPV vaccines significantly. For instance, as per the India, HPV and Related Cancers, 2023 report, cervical cancer ranks as the 2nd most frequent cancer among women in India and the 2nd most frequent cancer among women between 15 and 44. As per the same source, 123,907 women are diagnosed with cervical cancer in India each year. Therefore, the high burden of cervical cancer is expected to boost the demand for HPV vaccine during the forecast period.
The initiatives by the government to fund the HPV vaccines and rising screening and immunization programs are expected to boost the market growth. For instance, according to an article published in January 2023 in Vaccines Journal, the HPV vaccine is fully funded for children aged 11-14 years old and for 15-26-year-old females in Switzerland. Also, in March 2022, in Greece, a gender-neutral HPV vaccination program was launched.
Furthermore, in January 2021, the United States Department of Health and Human Services launched the "HPV VAX NOW" campaign to increase HPV vaccination rates among young adults aged 18-26 years. Also, in November 2021, Saudi Arabia launched a human papillomavirus infection program for young girls aged 9-13 years to prevent them from developing cervical cancer. This is expected to increase the development of HPV-cervical cancer vaccines hence bolstering the market growth.
Therefore, the factors such as the high burden of cervical cancer and rising screening and immunization programs, and launches of vaccines, the studied market is expected to witness growth during the forecast period. However, stringent regulation is likely to hinder market growth over the forecast period.
Human Papillomavirus Vaccine Market Trends
Cervical Cancer Segment is Expected to Hold the Significant Market Share Over the Forecast Period
The cervical cancer segment is expected to witness healthy growth in the market during the forecast period. Nearly all instances of cervical cancer are caused by human papillomavirus (HPV). The segment is expected to grow during the forecast period due to the growing burden of cervical cancer, supported by government initiatives for early screening, vaccination, and vaccine approval.According to the American Cancer Society (ACS) 2023 report, an estimated 13,960 new cases of cervical cancer are predicted to be diagnosed in the United States in 2023. Moreover, as per Japan Human Papillomavirus, Fact Sheet 2023, every year, 12,785 women are diagnosed with cervical cancer, with an incidence of HPV-related cervical cancer of 19.8 in 2023. Therefore, the high burden of cervical cancer will boost the demand for developing innovative vaccines, propelling market growth.
Furthermore, the rising screening and awareness initiatives increase the diagnoses, further expected to boost the market growth. For instance, in February 2022, the Department of Health and Social Care (DHSC), with the support of the National Health Service (NHS), launched a new national cervical screening campaign to boost the number of eligible candidates to attend their cervical screening in England. The increased screening is projected to boost the demand for the HPV vaccine to provide protection against the high-risk HPV types associated with cervical cancer, thereby propelling the segment's growth.
The rising research and development to launch innovative vaccines is projected to boost the market growth. For instance, in September 2022, the Government of India launched the Quadrivalent Human Papillomavirus vaccine (qHPV) against cervical cancer in India, which was developed by the Serum Institute of India (SII) and the Department of Biotechnology (DBT). As per the SII, the vaccine would cost around USD 2.5 - USD 5 (INR 200-400) per dose, which is significantly lower compared to other parts of the world.
Thus, owing to the increasing cases of cervical cancer, rising cervical cancer screening, and growing vaccine launches, the segment is expected to witness high growth over the forecast period.
North America is Expected to Hold Significant Market Share Over the Forecast Period
North America is expected to hold a significant market share of the HPV vaccine market owing to better healthcare infrastructure and the high burden of Cervical Cancer and other HPV-related cancers. In addition, the increased presence of market players and strategic initiatives also drive the market growth during the forecast period.According to the American Cancer Society 2022 report, 9,440 new anal cancer were diagnosed in the United States in 2022. Moreover, per the Canadian Cancer Society, 1,450 Canadian women were diagnosed with cervical cancer in 2022. Therefore, the high burden of such cancers is expected to boost the growth of the studied market in the region during the forecast period.
In addition, according to an article published in February 2023 in the Human Vaccine and Immunotherapeutic Journal, HPV vaccination in the United States can start at age nine and last until age 26. Also, approximately 77% of 13 to 17-year-old teenagers have received one or more doses of the HPV vaccine, and nearly 62% have finished the entire series. The high coverage for the HPV vaccine and early screening initiatives in the region boost the demand for HPV vaccines during the forecast period.
Furthermore, the strategic initiatives by market players, such as launches and approvals, partnerships, and collaborations, also accelerate the market growth. For instance, in April 2022, Merck Canada received Health Canada's approval for an expanded indication of GARDASIL 9 ((Human Papillomavirus 9-valent Vaccine, Recombinant) in individuals 9 through 45 years of age to prevent infection caused by the Human Papillomavirus (HPV) types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Therefore, factors such as the growing cancer burden and vaccine launches and approvals are expected to boost the market growth in North America during the forecast period.
Human Papillomavirus Vaccine Industry Overview
The market studied is a consolidated market owing to the presence of a few major market players. The competitive landscape includes an analysis of a few international as well as local companies which hold the market shares and are well known including Merck & Co., Inc., GSK plc, Serum Institute of India Pvt. Ltd., and Wantai BioPharm among others.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
1 INTRODUCTION
4 MARKET DYNAMICS
5 MARKET SEGMENTATION (Market Size by Value - USD)
6 COMPETITIVE LANDSCAPE
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Bharat Biotech
- Merck & Co., Inc.,
- GSK plc.
- Serum Institute of India Pvt. Ltd.
- Wantai BioPharm
- INOVIO Pharmaceuticals
- Walvax Biotechnology Co., Ltd
- Novartis AG
- Inovio pharmaceuticals
- AstraZeneca