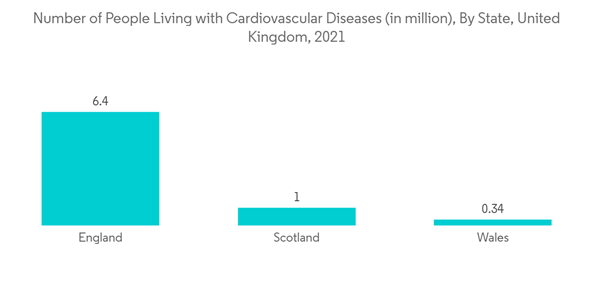
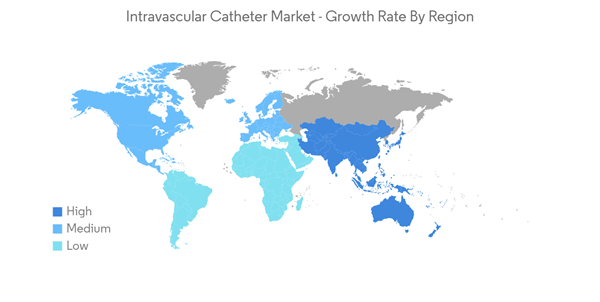
The COVID-19 pandemic significantly impacted the market studied. Due to the sudden onset of the pandemic, a short-term negative impact was initially seen on the market, owing to the decreased patient visits to hospitals and clinics. However, due to trials by scientists or researchers in intravenous catheterization and associated benefits among COVID-19 patients, the market witnessed considerable growth in the post-pandemic phase. As per a research article published in the Journal of Emergency Nursing in March 2022, when a nonrandomized clinical trial was conducted among COVID-19-infected patients in China, peripheral intravenous catheter (IC) placement using an infrared vein visualizer showed satisfactory results in a shortened procedure time. Thus, these instances during the later phases of the pandemic created a demand for intravenous catheterization, ultimately generating opportunities for the dives among end users. Therefore, even though the market experienced a setback initially due to its satisfactory outcome and clinical significance in the treatment of COVID-19 patients, the demand for the IC increased. Thus, the demand for ICs is expected to continue its stronghold and contribute to market growth over the coming years.
Factors such as increasing cases of target diseases, rapid improvement in IC technology, and a rising number of catheterization laboratories are anticipated to fuel the market growth during the forecast period.
The ICs allow access to the patient's bloodstream easily and reduce the need for needle sticks into the vein. Due to this, ICs have become vital medical devices for managing patients with severe and chronic diseases. The rise in vascular disease and the availability of technologically advanced products with better performance and clinical significance are a few other factors that are accelerating the studied market.
For instance, as per data released by FDA in January 2023, in the United States, heart disease affects more than 1 in 10 adults, or nearly 30 million, every year and is one of the major causes of mortality for people of most racial and ethnic groups. In addition, as per a research study published in Chemical Engineering Journal in April 2022, the increasing rate of hospitalization associated with the elevated prevalence of vascular diseases has accelerated the use of ICs. The ICs allow vascular diagnostics, injection of drug fluids, and delivery of other surgical devices. Hence, the increase in target disease globally is anticipated to increase the demand for ICs and is further expected to contribute to the overall market growth.
Furthermore, the launch of new products and technological advancements are predicted to drive the studied market growth. For instance, in September 2022, Wipro GE Healthcare launched its 'Made in India,' 'AI-powered' Cath lab-Optima IGS 320 to advance cardiac care in India and leverages the GE proprietary AutoRight technology. AutoRight is the first neural network-based interventional image chain featuring artificial intelligence. It automatically optimizes image and dose parameters in real time. Thus, such developments are likely to support market growth.
However, complications and side effects associated with catheters and a lack of reimbursements are predicted to hinder the market's growth.
Intravascular Catheter Market Trends
Short Peripheral Intravascular Catheters (PIVC) are Expected to Grow Significantly During the Forecast Period
Generally, a peripheral intravenous catheter (PIVC) is a thin plastic tube inserted into a vein using a needle. A short PIVC is a catheter of reduced length that is inserted in the forearm with its tip in a small-caliber vein. Additionally, short PIVCs are over-the-needle catheters that have a hollow metal needle positioned inside the catheter and are generally inserted in superficial veins. The short PIVCs are often called cannulas, venflons, IVs, or drips. These are used in most clinical settings.The advantages and benefits associated with the short PIVC are anticipated to gain demand among the end users. As per a study published in NursingOpen Journal in March 2022, when a standardized PIVC audit tool was developed to audit hospitalized patients requiring a PIVC insertion, the majority of audited short PIVC were inserted in a ward (57.6%), operating theatre (18.7%) or Emergency Department (ED) (11.6%) without a need for daily dressing on the insertion site. Thus, the high adoption of the devices is anticipated to contribute to the segment’s growth.
Additionally, the engagement of market players in product expansion is another factor that is expected to drive the growth of the segment over the forecast period. For instance, the availability of products such as BD Insyte-N Autoguard straight ICs, 14 mm long and the shortest on the market, accommodates small veins and provides a greater choice of insertion sites. Also, these are recommended for fragile veins and low venous pressure. Thus, the availability of such products in the market is anticipated to create competitiveness among the players and contribute to the segment’s growth.
North America is Expected to Hold a Significant Share of the Market
North America is expected to hold a major share of the market, and it is predicted to continue the same growth trend over the forecast period. The major factors fuelling the market growth in the region are the increasing prevalence of chronic diseases and the growing number of product approvals.Cardiovascular disease is one of the major causes of healthcare burden. According to the January 2023 update by the University of California, cardiovascular disease or disorders of blood vessels is the foremost cause of disability and mortality among men and women in the United States. In addition, as per July 2022 data released by Health Canada, about 1 in 12 (or 2.6 million) Canadian adults age 20 and over live with diagnosed heart disease every year. This high prevalence of diseases is anticipated to increase hospital admissions requiring treatments followed by vascular catheterization and vein access. Thereby, it is expected to drive market growth in the region.
Furthermore, several market players are engaged in implementing strategic initiatives such as product innovations and other activities, which are also contributing to the market's growth in the region. For instance, in April 2022, Shockwave Medical, a California-based company, launched its Shockwave M5+peripheral intravascular lithotripsy (IVL) catheter post receiving a CE mark and Food and Drug Administration (FDA) clearance. In addition, in July 2022, Intravascular Imaging Incorporated launched its 3 Fr NIRF-IVUS (Near-Infrared Fluorescence Intravascular Ultrasound) imaging catheter for human coronary imaging. In collaboration with the Massachusetts General Hospital, the catheter was designed and tested ex-vivo in human coronary arteries and in vivo in preclinical studies. These developments are expected to significantly contribute to the competitiveness in the market, augmenting the demand for ICs within the region. Hence, the market is expected to witness significant growth during the forecast period due to the abovementioned factors.
Intravascular Catheter Industry Overview
The market is highly competitive and comprises many players engaged in activities such as medical device manufacturing, raw material supply, sales and distribution, and many other services. Therefore there is an increase in the number of companies investing in emerging nations that drives the market growth in such regions. The market is also influenced by ongoing mergers and acquisitions among major market players. Boston Scientific Corporation, Becton, Dickinson and Company (C. R. Bard, Inc), B. Barun, Cook Medical, and McKesson Corporation are a few major players.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Boston Scientific Corporation
- Becton, Dickinson and Company (C. R. Bard, Inc)
- Medline Industries, Inc
- Cook Medical
- Edwards Life Sciences Corporation
- Johnson & Johnson
- McKesson Corporation
- Medtronic PLC
- Smiths Medical
- Terumo Corporation
- B. Barun
- Angiplast Pvt. Ltd.