Speak directly to the analyst to clarify any post sales queries you may have.
A precise and clinically oriented overview of how material innovations, regulatory rigor, and clinician demands are reshaping adhesive and sealant use across healthcare settings
The medical adhesives and sealants sector is experiencing a complex convergence of clinical need, material science innovation, and regulatory scrutiny. Across surgical subspecialties and medical device manufacturing, adhesives and sealants are increasingly relied upon to deliver faster procedures, reduce infection risk, and enable minimally invasive techniques that improve patient recovery trajectories. Innovations in chemistries and curing mechanisms have expanded application envelopes, while user-centric demands for ease of use, consistent performance, and storage stability continue to shape product design.Stakeholders from clinicians to procurement teams are demanding solutions that balance biocompatibility with handling characteristics and long-term performance. At the same time, cross-disciplinary research into biomimetic materials and bioresorbable chemistries is delivering new options for tissue repair and wound management. These developments are paralleled by heightened regulatory expectations for labeling clarity and post-market surveillance, which necessitate more robust clinical evidence and traceability across the supply chain. Consequently, companies are investing in clinical partnerships and iterative product validation to demonstrate both safety and procedural efficacy.
Together, these forces underscore a transition from commodity adhesives toward performance-differentiated products tailored to specific clinical workflows. This introduction positions readers to understand not only the technology trajectories but also the operational and regulatory imperatives that will determine adoption and commercialization success in the coming years.
Identification of the major disruptive clinical, technological, regulatory, and supply chain shifts that are redefining competitive advantage and product adoption in the industry
The landscape of medical adhesives and sealants is being transformed by several disruptive shifts that are altering competitive dynamics and value creation. First, the move toward minimally invasive procedures has elevated demand for adhesives that enable secure bonding in constrained anatomical environments while being compatible with endoscopic and robotic platforms. This clinical trend has prompted developers to optimize rheological properties and curing profiles to support rapid application and reliable fixation under challenging conditions.Second, advances in polymer science and biologic materials have enabled hybrid solutions that combine structural adhesion with bioactive functions such as hemostasis, antimicrobial delivery, or tissue regeneration. Such multifunctional products deliver differentiated clinical outcomes, and as a result, integration of cross-disciplinary R&D has become increasingly important. Third, regulatory pathways and post-market expectations have become more exacting; manufacturers are now expected to provide clearer safety dossiers and real-world performance data, necessitating stronger clinical partnerships and investment in evidence generation.
Finally, procurement and supply chain resilience have ascended to strategic importance, pushing firms to diversify suppliers, localize manufacturing, and build more transparent sourcing practices. Collectively, these shifts are converging to favor companies that can rapidly iterate product design, demonstrate clinical value through rigorous data, and secure resilient supply chains that withstand geopolitical and logistic pressures.
A rigorous assessment of how tariff changes enacted in 2025 have shifted procurement strategies, sourcing priorities, and manufacturing localization decisions across the industry
Tariff policy changes implemented in 2025 have introduced tangible friction into global supply chains for medical adhesives and sealants, prompting manufacturers and purchasers to reassess sourcing and pricing strategies. The immediate operational impact was an elevation in landed costs for imported raw materials and finished products, which has compelled firms to evaluate alternative procurement pathways and to consider partial localization of key production steps. These tariff effects were felt unevenly across product types and packaging formats, with high-value specialty chemistries and imported cartridge systems particularly exposed to increased import duties and logistical costs.In response, companies recalibrated supplier contracts, explored tariff engineering where feasible, and intensified negotiations to pass through only a portion of cost increases while protecting clinical customers from abrupt pricing shocks. Contract manufacturers and distributors played a critical role in absorbing near-term disruptions by offering flexible inventory arrangements and strategic warehousing options. Moreover, greater emphasis was placed on supplier diversification, including qualification of additional sources from tariff-exempt jurisdictions and ramping regional manufacturing capacity for high-demand items.
Longer term, the tariff environment has accelerated strategic conversations about vertical integration and localized manufacturing as a means to insulate critical supply lines. Regulatory and quality systems were also adapted to shorten qualification cycles for alternative materials and components. Taken together, these responses illustrate the sector’s capacity to adapt to policy-induced dislocations without compromising clinical supply continuity.
Comprehensive segmentation-driven insights that map technology, form factor, packaging, and application-specific requirements to clinical and commercial decision points
Insightful segmentation reveals where performance differentiation and commercial opportunity are concentrated across product and application dimensions. Based on Type, the market is studied across Adhesives and Sealants, which captures the foundational distinction between bonding technologies and barrier or fluid-control chemistries. Based on Application, the market is studied across Dental, Device Assembly, Hemostasis, Ophthalmic, Orthopedic, Tissue Repair, and Wound Closure, and these application areas illuminate divergent clinical requirements: device assembly further differentiates into Hard Devices and Soft Devices, hemostasis segments into Collagen-Based, Fibrin Sealant, and Synthetic Hemostat, tissue repair divides into Hard Tissue and Soft Tissue, and wound closure distinguishes between Suture Replacement and Tissue Adhesion Strips, each representing unique performance and regulatory pathways.Based on Form, the market is studied across Liquid, Paste, and Solid, reflecting differences in handling, dispensing equipment, and storage. Based on Packaging, the market is studied across Bulk, Cartridge, Sachet, and Tube, which directly influences supply chain logistics, clinician convenience, and single-use versus multi-use economics. Based on Technology, the market is studied across Heat Curing, Moisture Curing, Pressure Sensitive, Two Part Mix, and UV Curing, categorizing products by activation mechanism and compatibility with clinical workflows. Together, these segmentation lenses reveal where technical innovation, regulatory complexity, and procurement preferences intersect, enabling clearer prioritization for product development, clinical trials, and go-to-market investments.
Strategic regional analysis explaining how regulatory diversity, procurement mechanisms, and clinical adoption patterns vary across major global clusters and shape go-to-market choices
Regional dynamics condition adoption pathways and commercial strategies in distinct ways, with each geographic cluster presenting unique reimbursement, regulatory, and procurement environments. The Americas encompasses a mature reimbursement landscape and consolidated hospital procurement systems, where clinical evidence and vendor consolidation often determine purchasing decisions. As a result, product introductions in this region typically require robust clinical data and strong post-market support to gain clinician and purchaser trust.Europe, Middle East & Africa presents heterogeneity across national regulatory frameworks and healthcare funding models; this diversity necessitates localized evidence strategies and flexible pricing approaches. In several jurisdictions within this cluster, centralized procurement and regional tendering can accelerate adoption for competitively positioned products but also require careful alignment of logistics and regulatory dossiers. Meanwhile, Asia-Pacific is marked by fast-evolving clinical capacities, a growing number of locally based manufacturers, and a strong emphasis on cost-effectiveness; rapid uptake can be achieved by aligning product designs to regional procedural preferences and by supporting local clinical validation.
Understanding these regional characteristics allows manufacturers to prioritize launch sequences, tailor regulatory filings, and design distribution partnerships that reflect localized clinical pathways and buyer expectations. Strategic regional engagement that balances global evidence generation with local market adaptation enhances both adoption speed and long-term market durability.
An evidence-focused competitive overview highlighting how technological differentiation, clinical validation capabilities, and supply resilience drive sustainable advantage in the sector
Competitive positioning in this sector is driven by a combination of proprietary chemistries, clinical validation capabilities, and supply chain resilience. Leading firms differentiate through investments in advanced polymer science and biologically active formulations that deliver targeted clinical advantages such as enhanced hemostasis, tissue integration, or bioresorbability. Equally important are capabilities in device-level integration, where adhesives and sealants must interface with substrates and instruments in complex environments, demanding co-engineering with device manufacturers.Companies that excel also tend to maintain strong regulatory affairs teams and clinical research capabilities that can generate the evidence necessary for broad clinician acceptance. Operational strengths-such as flexible manufacturing systems, quality management excellence, and diversified supplier networks-reduce vulnerability to input shocks and regulatory audits. Additionally, partnerships between material suppliers, contract manufacturers, and clinical institutions accelerate product development timelines and facilitate real-world data collection. These collaborative models frequently unlock faster iterations and improved product-market fit than isolated, in-house development.
In sum, the competitive landscape favors organizations that integrate advanced material science with rigorous clinical validation and resilient operations, thereby enabling sustained product differentiation and consistent supply to healthcare customers.
Concise, actionable recommendations for executives to align R&D, regulatory strategy, and supply chain resilience with clinician needs to accelerate adoption and protect margins
Leaders seeking to convert insight into competitive advantage should pursue a set of targeted, actionable strategies that address clinical, regulatory, and commercial dimensions simultaneously. Focused investment in clinically meaningful product features-such as reduced cure time, targeted bioactivity, or simpler application-should be paired with early-stage clinician collaboration to validate usability under real procedural conditions. Parallel investment in controlled evidence generation will improve reimbursement discussions and clinician adoption while reducing time-to-decision in hospital procurement committees.Operationally, diversifying raw material suppliers and exploring regional manufacturing partnerships will mitigate tariff and logistics exposures. Where tariffs or import constraints are material, selectively localizing manufacturing for high-risk inputs can safeguard continuity and reduce total landed costs. On the regulatory front, building robust post-market surveillance systems and establishing clear labeling and instructions for use will streamline approvals and strengthen buyer confidence. Commercially, offering training programs and procedural support to clinicians can accelerate adoption by reducing perceived implementation risk.
Together, these actions create a virtuous cycle: clinically validated, easy-to-use products supported by resilient operations and thoughtful commercial engagement are more likely to achieve sustained penetration and to command premium positioning within complex procurement environments.
A transparent explanation of the mixed-methods research approach that integrates expert interviews, technical review, and cross-validation to ensure actionable and reliable sector insights
The research underpinning this analysis combines qualitative and quantitative methodologies designed to produce robust and actionable insights. Primary inputs included structured interviews with clinicians, procurement professionals, and R&D leaders across surgical and device manufacturing domains, supplemented by technical reviews of recent peer-reviewed literature and regulatory filings. The approach emphasized triangulation: qualitative perspectives informed hypothesis generation, which were then tested against secondary technical documentation and vendor disclosures to validate emergent themes.The analytical framework segmented the market across product type, application, form, packaging, and activation technology to ensure that conclusions were sensitive to differences in clinical workflows and supply chain characteristics. Attention was paid to regulatory pathways and post-market surveillance requirements to reflect the practical constraints manufacturers face during commercialization. Data integrity checks included cross-referencing interview inputs with publicly available regulatory actions and clinical trial registries to ensure factual accuracy.
Limitations of the study are acknowledged and include potential variability in regional procurement practices and the evolving nature of regulatory guidance in certain jurisdictions. To mitigate these limitations, the methodology incorporated iterative validation with external experts and scenario analysis to test the robustness of strategic conclusions under alternative regulatory and supply chain conditions.
A conclusive synthesis that ties together clinical trends, material innovation, and operational resilience to define the strategic imperatives for successful market participation
In conclusion, the medical adhesives and sealants sector stands at an inflection point where material innovation, clinical demand for minimally invasive solutions, and heightened regulatory expectations converge. Companies that can integrate advanced functional chemistries with rigorous clinical validation and resilient operations will be best positioned to capture sustained clinical adoption and commercial value. Procurement disruptions and policy shifts have underscored the importance of supply chain agility, making supplier diversification and selective localization pragmatic strategic priorities.Clinicians and healthcare purchasers increasingly expect products that not only meet safety and efficacy benchmarks but also simplify procedural workflows and reduce downstream complications. Consequently, product strategies should emphasize not just technical performance but also usability, clinician training, and evidence of long-term outcomes. Organizations that can translate technical differentiation into clear procedural advantages and align those claims with robust clinical data will gain preferential access to consolidated procurement channels and premium positioning.
Ultimately, success in this evolving landscape requires an integrated approach that aligns material science innovation, regulatory foresight, clinical engagement, and operational excellence to deliver safe, effective, and commercially viable adhesive and sealant solutions.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Medical Adhesives & Sealants Market
Companies Mentioned
The key companies profiled in this Medical Adhesives & Sealants market report include:- 3M Company
- Arkema S.A.
- Avery Dennison Corporation
- B. Braun SE
- Dow Inc.
- Evonik Industries AG
- H.B. Fuller Company
- Henkel AG & Co. KGaA
- Johnson & Johnson
- Sika AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
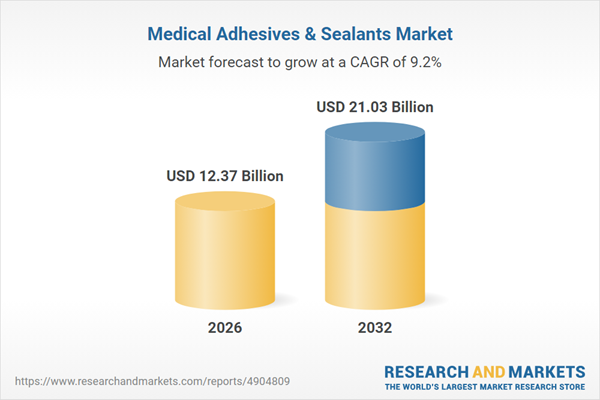
| Estimated Market Value ( USD | $ 12.37 Billion |
| Forecasted Market Value ( USD | $ 21.03 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |