This study focuses on the market side of iPSCs rather than the technical side. Different market segments for this emerging market are covered. For instance, product function-based market segments include molecular and cellular engineering, cellular reprogramming, cell culture, cell differentiation, and cell analysis. Application-based market segments include drug development and toxicity testing, academic research, and regenerative medicine. iPSC-derived cell type-based market segments include hepatocytes, neurons, cardiomyocytes, endothelial cells, and other cell types. Other cell types are comprised of astrocytes, fibroblasts, and hematopoietic and progenitor cells, among other substances. Geographical-based market segments include the U.S., Asia-Pacific, Europe, and the Rest of the World. The research and market trends are also analyzed by studying the funding, patent publications, and research publications in the field.
This report focuses on the market size and segmentation of iPSC products, including iPSC research and clinical products. The market for iPSC-related contract services is also discussed. iPSC research products are defined as all research tools, including iPSCs and various differentiated cells derived from iPSCs, various related assays and kits, culture media and medium components (e.g., serum, growth factors, inhibitors), antibodies, enzymes, and products that can be applied for the specific purpose of executing iPSC research. For this report, iPSC products do not cover stem cell research and clinical products that are broadly applicable to any stem cell type.
This report discusses key manufacturers, technologies, and factors influencing market demand, including the driving forces and limiting factors of the iPSC market’s growth. Based on these facts and analysis, the market trends and sales for research and clinical applications are forecast through 2028. One particular focus on the application of iPSCs was given to drug discovery and development, which includes pharmaco-toxicity screening, lead generation, target identification, and other preclinical studies; body-on-a-chip; and 3D disease modeling. Suppliers and manufacturers of iPSC-related products are discussed and analyzed based on their market shares, product types, and geography. An in-depth patent analysis and research funding analysis are also included to assess the overall direction of the iPSC market.
Detailed technologies such as those for generating iPSCs, differentiating iPSCs and controlling the differentiation, and large-scale manufacturing of iPSCs and their derivative cells under Good Manufacturing Practice (GMP) compliance or xeno-free conditions are excluded from the study. They are beyond the scope of this report.
The induced pluripotent stem cell market has been analyzed for four main geographic regions: The U.S., Europe, Asia-Pacific, and the Rest of the World (RoW). The report will provide details with respect to induced pluripotent stem cells.
Report Includes
- 34 data tables
- An overview of the global market for induced pluripotent stem cells (iPSCs)
- Analysis of global market trends, featuring historical revenue data for 2021 and 2022, estimated figures for 2023, as well as forecasts for 2028, including projections of compound annual growth rates (CAGRs) through 2028
- Evaluation of the current market size and revenue growth prospects, accompanied by a market share analysis by reprogramming method, method, application, derived tissue cell type, species, product function, end use, and geographic region
- Analysis of the market’s dynamics, including drivers, restraints and opportunities, and the regulatory environments impacting the market
- Assessment of R&D activity in the iPSC segment, as well as new developments and pipeline products
- Information on the latest research activities, emerging technologies, and clinical trials
- Market share analysis of the key companies and coverage of mergers & acquisitions, joint ventures, collaborations, and partnerships
- Profiles of the major players in the industry
Table of Contents
Companies Mentioned
- Addgene
- Allele Biotechnology And Pharmaceuticals Inc.
- Alstem
- Applied Biological Materials Inc. (Abm)
- Applied Stemcell Inc.
- ATCC
- Axol Bioscience Ltd.
- Bio-Techne Corp.
- Bluerock Therapeutics
- Bristol Myers Squibb (Ipierian)
- Cell Signaling Technology (Cst)
- Corning Inc.
- Creative Bioarray
- Fate Therapeutics
- Fujifilm Cellular Dynamics Inc. (Fcdi)
- Genecopoeia Inc.
- Gentarget Inc.
- ID Pharma Co. Ltd.
- Invivogen
- Lonza Group Ltd.
- Megakaryon Corp.
- Merck Kgaa
- Ncardia
- Newcells Biotech
- Peprotech Inc.
- Plasticell Ltd.
- Promega Corp.
- Promocell Gmbh
- Qiagen Nv
- Reprocell Inc.
- Sciencell Research Laboratories
- Stemcell Technologies
- System Biosciences Inc.
- Takara Bio Usa Inc. (Clontech Laboratories)
- Thermo Fisher Scientific
- Vertex Pharmaceuticals Inc.
- Waisman Biomanufacturing
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 121 |
| Published | January 2024 |
| Forecast Period | 2023 - 2028 |
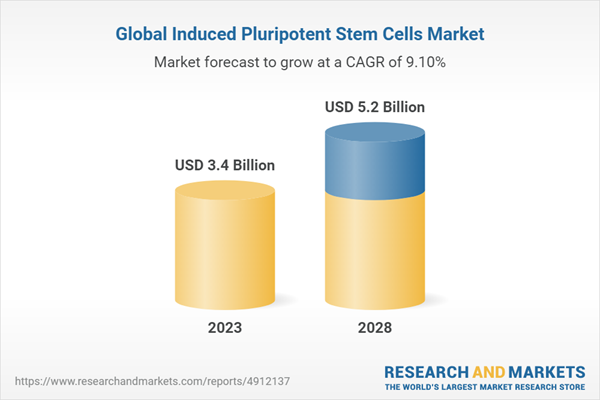
| Estimated Market Value ( USD | $ 3.4 Billion |
| Forecasted Market Value ( USD | $ 5.2 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 37 |