COVID-19 had a significant impact on the cardiac prosthetic devices market owing to delayed cardiac consultation and surgical procedures. Moreover, many complications, such as prosthetic valve failure, were reported in COVID-19-infected patients. For instance, as per the article published in December 2022 in Springer, due to the hypercoagulability associated with COVID-19 infection, the risk of prosthetic valve failure and valve thrombosis was found to be more prone in hospitalized COVID-19-infected patients. However, in the post-pandemic situation, as the restriction was lifted, the market is expected to witness significant growth due to the increase in post COVID cardiac complications and the rise in technological advancements in cardiac prosthetic devices.
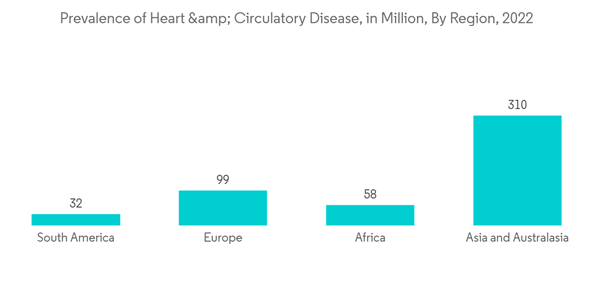
The major factors attributing to the growth of the market are the rise in the prevalence of multiple cardiovascular indications, atrial fibrillation, cardiac valve failure, and stroke. For instance, as per the data from the CARES report 2021, 97.6% of adults and 2.4% of children were reported with out-of-hospital cardiac arrest in 2021 in the United States, and the median age of OHCA is considered to be 64 years. Hence, prosthetic devices are widely utilized to cope with the rising prevalence of cardiac complications globally and support cardiac health for a longer period, thereby driving market growth. Furthermore, the rise in the global geriatric population is likely to increase the market growth since cardiac complications rise with age. For instance, according to the World Population Prospects 2022 report published by the United Nations, the global geriatric population is increasing rapidly around the world, and between 2022 and 2050, the older population is projected to grow at rates above 3% per year in Northern Africa and Western Asia, sub-Saharan Africa, Oceania (excluding Australia and New Zealand) and Central and Southern Asia.
Moreover, product launches and strategic initiatives by the key players are expected to drive market growth over the forecast period. For instance, in June 2021, Medtronic launched Micra AV, a miniaturized, fully self-contained pacemaker that delivers advanced pacing technology to atrioventricular (AV) block patients via a minimally invasive approach. The device can sense atrial activity without a lead or device in the upper chamber of the heart. Thus, developments in medical devices like pacemakers are expected to increase in demand for cardiac prosthetic devices and lead to market growth over the forecast period.
Hence, due to the rise in cardiac complications, the increase in the geriatric population, and the surge in cardiac prosthetic device launches by the key players, the studied market is expected to drive market growth over the forecast period. However, the strict regulatory framework and the high cost of implantation, and the risk of repetitive surgeries are likely to restrain the market growth.
Cardiac Prosthetic Devices Market Trends
Mechanical Valves are Expected to Hold the Significant Market Share in the Cardiac Prosthetic Devices Market.
The mechanical valves are expected to attribute a significant market share in the studied market owing to their lifetime solution for the patient due to their mechanical strength and flexibility of durable raw polymeric materials used in their manufacturing process. Furthermore, growing preference by physicians and patients due to the lower risk of clinical problems compared to other valves, as well as the reduced need for repetitive surgeries with these valves, contribute to the rise in adoption of these systems, propelling the market growth.Additionally, many research activities are ongoing to understand the advantages and future opportunities provided by mechanical heart valves. For instance, as per the article published in May 2022 in PubMed, each surgical and transcatheter technique has advantages such as improved long-term durability with surgically installed mechanical valves. Hence, such advantages offered by the mechanical valve are expected to increase the demand and thereby drive segment growth over the forecast period. Furthermore, the rise in mechanical valve product approvals and launches by the key players is expected to drive market growth over the forecast period. For instance, in September 2021, Abbott received FDA approval for the company's Epic Plus and Epic Plus Supra Stented Tissue Valves to improve therapy options for people with aortic or mitral valve disease. These devices build off Abbott's Epic surgical valve platform and include innovations that make implantation of the valve and future cardiac interventions easier. The Epic Plus Stented Tissue Valves are the latest addition to Abbott's portfolio of surgical tissue and mechanical heart valves.
Hence, due to the rise in mechanical valve product launches by the key players and the increase in advantages offered by mechanical valves in cardiac surgeries, the studied segment is expected to drive market growth over the forecast period.
North America Anticipated to Hold a Significant Share in the Market Over the Forecast Period
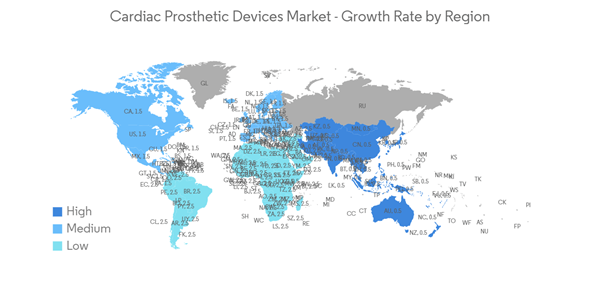
North America is expected to witness significant growth in the cardiac prosthetic devices market throughout the forecast period due to the rising prevalence of cardiovascular disorders and the increase in product launches by the key players. For instance, according to the CIHI July 2022 report, about 2.4 million Canadians have heart disease, and collaboration between CIHI and CCS on the CCQI project has contributed to monitoring and improvements in key areas related to cardiac care interventions across Canada. Hence, such initiatives occurring across the studied region are expected to boost market growth over the forecast period.Moreover, product launches, strategic partnerships, and acquisitions by the key players are expected to increase the market growth in the studied region over the forecast period. For instance, in April 2022, Abbott received FDA approval for its Aveir single-chamber (VR) leadless pacemaker for the treatment of patients in the U.S. with slow heart rhythms. This marks a significant advancement in patient care and brings new, never-before-seen features to patients and their physicians.
Also, increasing awareness programs by healthcare associations and manufacturers by promoting positive health and the advantages of technologically advanced prosthetics, along with the availability of advanced healthcare infrastructure in the region, contributes to its outstanding share in the global market revenue. For instance, in February 2022, Montreal Heart Institute Foundation (MHIF) launched a vast awareness campaign about the importance of research in the fight against cardiovascular disease.
Hence, due to the rise in the prevalence of cardiovascular diseases and the increase in product launches and awareness campaigns, North America is expected to witness a significant share of the market over the forecast period.
Cardiac Prosthetic Devices Market Competitor Analysis
The cardiac prosthetic devices market is moderately fragmented and consists of several major players. Most of the key players currently dominating the market are focusing on technological advancements to address the unmet needs of cardiac diseases with the enhanced safety of cardiac prosthetic devices resulting in demand for the market. Some of the companies which are currently dominating the market are Abbott Laboratories, LivaNova PLC, Medtronic plc, Boston Scientific Corporation, and Edwards Lifesciences Corporation.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- LivaNova PLC
- Medtronic plc
- Boston Scientific Corporation
- Edwards Lifesciences Corporation
- Colibri Heart Valve
- Meril Life Sciences Pvt Ltd
- Biotronik
- Lepu Medical Technology Co Ltd
- Siemens Healthineers