The COVID-19 pandemic had a significant impact on both the global economy and healthcare infrastructure. The pediatric neuroblastoma treatment market was severely impacted due to a disruption in medical supplies and delayed fulfillment of neuroblastoma treatment medications and treatments. For instance, an article published in January 2021 in PubMed stated that there was a significant reduction in the daily mean number of pediatric patients undergoing chemotherapy, radiotherapy, surgery, and imaging studies during COVID-19. However, the market is anticipated to witness growth in the coming years due to the rise in the burden of pediatric neuroblastoma and the increase in research and development for pediatric neuroblastoma treatments.
According to a National Cancer Institute (NCI) November 2021 update, among children (ages 0 to 14 years), the most common types of cancer are leukemias, followed by brain and other CNS tumors, lymphomas, neuroblastoma, kidney tumors, and malignant bone tumors. As per the same source, in 2021, it was estimated that 15,590 children and adolescents ages 0 to 19 were likely to be diagnosed with cancer.
Additionally, the government's increased investments in R&D initiatives to build the pediatric medical infrastructure and the public's growing knowledge of pediatric neuroblastoma therapy are promoting market expansion. For instance, the Rally Foundation for Childhood Cancer Research granted USD 3.4 million in grants to Drs. Miller Huang and Satyaki Sengupta in March 2022 to study the role of chromosome 17q gain in neuroblastoma and Dr. Muxiang Zhou to find novel MYCN inhibitors of therapy for pediatric neuroblastoma, and other researchers.
On the other hand, the high cost and side effects associated with cancer therapy are likely to restrain the market growth over the forecast period.
Pediatric Neuroblastoma Treatment Market Trends
TheChemotherapy Segment is Anticipated to Witness a Growth in the Pediatric Neuroblastoma Treatment Market Over the Forecast Period
Anti-cancer medications are used in chemotherapy, and they are often injected into a vein. To find and kill cancer cells, the medications enter the bloodstream and circulate throughout the body. Chemotherapy can therefore be used to treat neuroblastoma that has spread to the liver, lungs, lymph nodes, bone marrow, liver, or other organs. The effectiveness of chemotherapeutic medications in the treatment of neuroblastoma as well as the rising incidence of the disease in the pediatric population, is primarily responsible for the segment's increasing growth rate.In addition, chemotherapy is the preferred method of treatment to lessen symptoms and decrease the spread of the disease. For better outcomes, it is also used in conjunction with other therapies like radiation therapy or surgery. For instance, in June 2021, the Memorial Sloan Kettering Cancer Center sponsored a clinical study that focused on determining whether N9 was a secure and efficient treatment for kids with neuroblastoma. Cyclophosphamide, topotecan, and vincristine (CTV), ifosfamide, carboplatin, etoposide (ICE), and cyclophosphamide, doxorubicin, and vincristine are the three chemotherapy drug combinations that make up the N9 regimen (CDV). Furthermore, as per an article published in June 2022 in the ASCO journal, the administration of dinutuximab (DIN) and sargramostim (GM-CSF) to Children’s Oncology Group (COG) chemotherapy cycles for 3-5 for patients with newly-diagnosed high-risk neuroblastoma was tolerable and feasible.
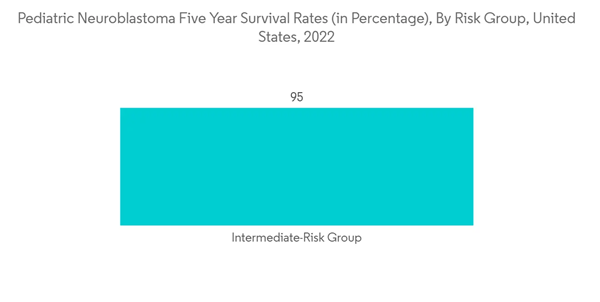
Chemotherapy is used to treat certain neuroblastoma patients in children, either before or after surgery. Chemotherapy is the primary treatment in a few other situations, particularly when cancer has progressed too far for surgery to completely eradicate it. The market being investigated will also develop as a result of increasing investments in researching the effectiveness of chemotherapy in treating pediatric neuroblastoma. For instance, Jason Shohet, MD, Ph.D., was awarded a new, two-year USD 300,000 Scholar Hope Grant in January 2022 to research overcoming drug resistance in chemotherapy treatments for neuroblastoma. This new grant is anticipated to focus on understanding the best way to combine enzyme PRMT5 targeting with established treatments. Furthermore, as per an American Cancer Society 2022 update, the 5-year survival rate for neuroblastoma in children under age 15 is 82%. For children with low-risk neuroblastoma, the 5-year survival rate is higher than 95%. For children with intermediate-risk neuroblastoma, the 5-year survival rate is between 90% and 95%. For children with high-risk neuroblastoma, the the-5-year survival rate is around 50%. For low-risk and intermediate-risk cases most of the survival is aided by proper chemotherapy and other adjuvant therapies. Hence, due to this, the adoption of chemotherapy is likely to increase and thereby boosting market growth.
North America is Likely to Witness a Growth in the Pediatric Neuroblastoma Treatment Market and Expected to do Same Over the Forecast Period
North America is expected to witness growth in the market owing to factors such as the high frequency of pediatric neuroblastoma in the area and the well-established healthcare infrastructure. In addition, encouraging government initiatives and a rise in research collaboration numbers are other factors anticipated to boost market expansion. Due to its pro-healthcare legislation, large patient population, and developed healthcare market, the United States is anticipated to have the largest share in this region.As per an American Society of Clinical Oncology (ASCO) February 2022 update, in North America, neuroblastoma affected 700 to 800 children annually between the ages of 0 and 14. 6%. The same source states that children under the age of five account for roughly 90% of neuroblastoma cases. The typical age of diagnosis is between one and two years.
Additionally, product approvals in the market are anticipated to broaden the competitors' product portfolios and increase effective treatment, which is likely to fuel market expansion. For instance, in June 2022, the FDA granted Omblastys (omburtamab) a priority review for a Biologics License Application for the treatment of children with central nervous system or leptomeningeal metastasis (a disease that spreads to the cerebrospinal fluid that surrounds the brain and spinal cord) from neuroblastoma.
Additionally, growing initiatives by important market participants, including collaborations, mergers, and acquisitions, among others, will also significantly contribute to the market's expansion. For instance, in August 2021, United Therapeutics Corporation entered into a partnership with former NFL player Devon Still and his daughter Leah, a high-risk neuroblastoma survivor, to introduce the educational program "Braving NeuroBLASToma," which increased the awareness of the uncommon cancer that affects neuroblasts, which are immature nerve cells.
Pediatric Neuroblastoma Treatment Industry Overview
The pediatric neuroblastoma treatment market is moderately competitive and consists of several major players. Some companies currently dominating the market are United Therapeutics Corporation, APEIRON Biologics AG, Baxter, CELLECTAR BIOSCIENCES, INC, Pfizer, Inc., Bayer AG, MacroGenics, Inc., and Sartorius AG. Product launches, partnerships or collaboration, and continuous investment in research and development are some strategies companies are adopting to enhance their market share.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- United Therapeutics Corporation
- APEIRON Biologics AG
- Baxter International
- Pfizer, Inc.
- Bayer AG
- MacroGenics, Inc.
- Sartorius AG
- CELLECTAR BIOSCIENCES, INC.
- PROVECTUS BIOPHARMACEUTICALS, INC.
- Y-mAbs Therapeutics, Inc.
- Amgen
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd