Speak directly to the analyst to clarify any post sales queries you may have.
A compelling introduction to the contemporary cell therapy environment where scientific maturation, industrialization, and regulatory clarity are converging to enable clinical translation
Cell therapy technologies are entering a phase where scientific advances, manufacturing maturation, regulatory clarity, and commercial ambition are converging to accelerate translation from lab to clinic. This introduction frames the contemporary landscape by emphasizing how improvements in vector design, cellular engineering, and process analytics are reducing technical barriers that historically constrained reproducibility and scale. At the same time, an expanding cadre of clinical programs across immuno-oncology, regenerative medicine, and neurodegeneration is generating a richer set of safety and efficacy signals that inform next-generation product development.Moreover, the industry is moving beyond proof-of-concept to a focus on industrialization: consistent raw material quality, closed-system manufacturing, and automated quality control are becoming prerequisites for broad clinical adoption. These operational shifts are accompanied by evolving regulatory expectations that prioritize product characterization and control strategy over descriptive batch testing, thereby enabling more predictable paths to approval. Together, these dynamics underscore why strategic leaders must reconcile scientific promise with practical delivery capabilities and why cross-functional integration across R&D, manufacturing, and regulatory teams is essential for translating cell therapies into durable commercial offerings.
A review of the transformative scientific, manufacturing, and commercial shifts redefining how cell therapies are developed, produced, and delivered to patients
The landscape of cell therapy is being reshaped by several transformative shifts that extend from foundational science to commercialization models. Advances in genome editing, single-cell characterization, and synthetic biology have expanded the range of manipulable cell types and enabled precise functional tuning, which in turn has broadened therapeutic applications and raised the bar for analytical characterization. Parallel to these scientific developments, digitalization and software-driven process control are enabling tighter manufacturing tolerances and real-time release strategies that significantly reduce time-to-dose uncertainties.In addition, stakeholder expectations have evolved: payers and health systems are increasingly focused on demonstrable long-term value and predictable supply reliability, prompting firms to design evidence-generation strategies that extend beyond initial clinical endpoints. Supply chain resilience has grown to strategic prominence, catalyzing regional manufacturing hubs and outsourced production partnerships that prioritize scalability and regulatory alignment. Taken together, these shifts are producing an industry in which multidisciplinary competence, strategic partnerships, and platform-level thinking are central to capturing the next wave of therapeutic opportunities.
An analysis of how 2025 tariff measures have reshaped sourcing, manufacturing footprint decisions, and supply chain resilience strategies across the cell therapy value chain
The introduction of new tariff measures in 2025 affecting biologics-related inputs has materially altered the operational calculus for cell therapy developers, contract manufacturers, and suppliers. Increased duties on certain reagents, equipment components, and critical consumables have pressured historically global supply chains, prompting firms to re-evaluate sourcing strategies and inventory policies. In practice, procurement teams are prioritizing qualification of alternate suppliers, increasing onshore inventory buffers where feasible, and accelerating vendor diversification to reduce exposure to single-origin risks.Consequently, capital allocation decisions are increasingly factoring in supply chain localization and manufacturing footprint redundancy. Companies pursuing in-house scale-up are weighing higher near-term capital expenditures for domestic manufacturing capacity against the recurring cost implications of tariffs on imported goods. For many organizations, the tariffs have catalyzed strategic partnerships with regional contract development and manufacturing organizations that can provide localized supply continuity and regulatory alignment. These responses, while adaptive, also introduce complexity in validation timelines and may require enhanced control strategies to ensure product comparability across sites. Ultimately, tariff-driven adjustments are reshaping procurement, operational resilience, and the economics of industrialization for the cell therapy sector.
A layered segmentation perspective revealing how offerings, therapy modalities, manufacturing processes, delivery methods, applications, and end users collectively determine strategic priorities for stakeholders
Segmentation offers a practical lens to understand where technological investment, regulatory effort, and clinical focus are concentrated across the cell therapy ecosystem. Based on offering, the industry infrastructure is structured around consumables, equipment, and software and services, with equipment investments centering on bioreactors and cell analyzers while software and services investments are focused on data management and quality control solutions that enable compliant, scalable operations. This offering perspective shows why companies that integrate hardware, consumable supply, and digital process control gain operational advantages in reproducibility and throughput.Based on therapy type, portfolios span allogeneic cell therapies, autologous cell therapies, and gene-modified cell therapies. Within these therapy-type categories, developers and clinicians are selecting cellular platforms to match biological attributes and clinical logistics: allogeneic approaches including dendritic cells, induced pluripotent stem cells, and natural killer cells favor off-the-shelf scalability; autologous approaches such as hematopoietic stem cells, mesenchymal stem cells, and T-cells emphasize individualized manufacturing and chain-of-identity controls; and gene-modified modalities like CAR-T and TCR therapies blend complex genetic modification with stringent release testing. This therapy-type segmentation clarifies why manufacturing strategies and regulatory expectations diverge significantly across programs.
Based on manufacturing process, distinct technical pathways include cell culture, cell expansion, and cell separation, where cell culture choices between 2D and 3D systems influence scalability and phenotype maintenance, cell expansion approaches favor adherent or suspension modalities depending on cell biology, and separation methods rely on fluorescence-activated or magnetic-activated sorting to achieve required purity. Based on delivery method, ex vivo and in vivo routes drive different regulatory and logistical imperatives, with ex vivo workflows emphasizing closed-system processing and cold-chain logistics, and in vivo strategies prioritizing vector safety and biodistribution analytics. Finally, based on application, therapeutic focus ranges across cardiovascular disease, musculoskeletal disorders, neurodegenerative diseases, and oncology, with sub-specialization such as ischemic and peripheral vascular targets, osteoarthritis and rheumatoid arthritis indications, Alzheimer’s and Parkinson’s disease approaches, and hematological and solid tumor oncology programs targeting specific tumor types including breast, lung, and prostate. Based on end user, the ecosystem serves biopharmaceutical companies, hospitals, and research institutes where hospitals differentiate by private versus public settings and research institutes segregate government research centers from university academic programs. Together, these layered segmentation lenses reveal why strategy must be tailored to therapeutic, technical, and end-user realities rather than relying on one-size-fits-all playbooks.
An assessment of how regional regulatory frameworks, manufacturing capacity, and investment dynamics in the Americas, Europe Middle East & Africa, and Asia-Pacific are shaping strategic rollouts
Regional dynamics are shaping where investment, manufacturing scale-up, and clinical development activities concentrate, with distinct competitive advantages emerging across major geographies. In the Americas, a robust ecosystem of venture capital, translational research institutions, and contract manufacturers supports rapid translational pathways, while regulatory engagement emphasizes predictable review timelines and evidence generation to support reimbursement discussions. This environment encourages partnerships between innovators and larger industry players to streamline late-stage development and commercialization pathways.In Europe, Middle East & Africa, regulatory harmonization efforts and capacity-building initiatives are fostering an increasingly coordinated approach to clinical access and manufacturing standards, even as national-level reimbursement systems require localized evidence strategies. The region’s strength in academic translational science and growing network of specialized manufacturing sites positions it as a complementary innovation hub. Across Asia-Pacific, rapid investment in biomanufacturing infrastructure, supportive policy frameworks for advanced therapies, and expanding clinical trial capacity are accelerating adoption, with several national programs incentivizing domestic production and technology transfer to reduce dependence on external supply.
These regional contrasts influence strategic decisions about where to locate pivotal trials, manufacturing capacity, and commercial launch sequences. Accordingly, organizations designing global rollouts must balance regulatory timelines, local reimbursement landscapes, and logistics to optimize patient access and operational continuity across these diverse geographies.
Insightful characterization of company strategies showing how platform builders, vertically integrated developers, specialized service providers, and digital enablers are shaping competitive dynamics
Key company behaviors are revealing clear strategic archetypes as organizations vie to capture clinical and commercial value within the cell therapy domain. Some firms are concentrating on platform technologies that can be applied across multiple therapy types, investing heavily in scalable bioreactor designs, closed-system automation, and integrated analytics to minimize per-dose variability. Other companies are adopting vertical integration strategies, moving from discovery through clinical manufacturing to commercial supply in order to retain control over critical quality attributes and supply continuity.A second archetype emphasizes specialized service models: contract developers and manufacturers are deepening therapeutic expertise to serve niche indications and to provide technology transfer capabilities that accelerate sponsor timelines. Simultaneously, software and data companies are building regulatory-grade data management systems and quality control solutions that facilitate compliance and enable process-intensification opportunities. Across the competitive landscape, strategic collaborations, licensing arrangements, and targeted M&A activity are common, as organizations seek to combine scientific differentiation with manufacturing know-how and market access capabilities. These patterns indicate that success will favor entities that align technical depth with pragmatic commercialization and supply strategies.
Clear and actionable recommendations for leaders to strengthen process control, supply resilience, evidence strategies, partnerships, and cross-functional capabilities to scale cell therapies
Industry leaders can take several actionable steps to convert scientific promise into reproducible patient impact and sustainable business models. First, invest in platform-level process control that unifies analytics, equipment, and data management to reduce batch variability and accelerate regulatory submissions. By harmonizing hardware and software stacks, organizations can achieve more predictable manufacturing performance and simplify comparability across sites. Second, prioritize supply chain resilience by qualifying alternate vendors, regional sourcing, and validating materials for interchangeability to mitigate tariff and logistics risks that can disrupt clinical timelines.Third, align evidence-generation strategies with payer expectations by designing longer-term follow-up and real-world evidence collection into pivotal programs; this enhances the value proposition for reimbursement discussions and supports broader adoption. Fourth, pursue strategic partnerships that pair scientific differentiation with manufacturing scale, whether through targeted alliances with contract developers and manufacturers or through shared infrastructure models that lower capital burden and accelerate time to dose. Finally, cultivate cross-functional capability within organizations-bringing together biology, process engineering, quality, regulatory, and commercial expertise-to ensure decisions made in discovery persist through to scalable, compliant manufacturing and market access.
A transparent explanation of the mixed-method research approach that integrates expert interviews, regulatory literature review, and iterative validation to produce actionable cell therapy insights
The research behind these insights combined primary and secondary qualitative methods to ensure a robust and triangulated understanding of technical, regulatory, and commercial dynamics. Primary inputs included structured interviews with senior leaders across development, manufacturing, regulatory affairs, and hospital administration, as well as direct consultations with technical experts in bioprocess engineering and analytical characterization. These engagements provided real-world perspectives on operational challenges, validation timelines, and procurement realities that shape program decisions.Secondary research involved systematic review of regulatory guidance documents, peer-reviewed literature on cellular engineering and process technologies, and industry conference proceedings to capture evolving standards and technological milestones. The analysis applied a cross-sectional segmentation framework that considered offering, therapy type, manufacturing process, delivery method, application, and end-user perspectives to generate layered insights. Throughout, findings were validated through iterative expert review cycles to ensure clarity, relevance, and practical applicability for decision-makers seeking to translate scientific advances into operational and commercial outcomes.
A concise conclusion connecting technological progress, operational challenges, regulatory evolution, and the imperative for integrated strategies to realize durable clinical impact
In conclusion, the cell therapy field stands at an inflection point where technical maturation, manufacturing industrialization, and evolving regulatory and payer expectations are collectively enabling broader clinical translation. While scientific advances have expanded the scope of what is biologically achievable, the enduring challenge lies in operationalizing those advances into reliable, scalable, and economically viable therapies. The combined pressures of supply chain dynamics, regional regulatory nuance, and the need for robust long-term evidence require organizations to adopt integrated strategies that span discovery, manufacturing, and commercialization.Forward-looking entities will prioritize platform resilience, data-driven process control, and collaborative models that distribute risk while preserving technical differentiation. With careful alignment of technical capabilities, regulatory strategy, and commercial planning, cell therapies can transition from pioneering treatments into sustainable standards of care across a growing set of indications. The imperative for leaders is to translate the current momentum into repeatable, patient-centric delivery systems that stand up to the complexities of global deployment.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Cell Therapy Technologies Market
Companies Mentioned
The key companies profiled in this Cell Therapy Technologies market report include:- Allogene Therapeutics Inc.
- Atara Biotherapeutics, Inc.
- Century Therapeutics, Inc.
- Charles River Laboratories International, Inc.
- Gamida Cell Ltd.
- GE HealthCare
- Gilead Sciences, Inc.
- Johnson & Johnson Services, Inc.
- Novartis AG
- Sartorius AG
- Takara Bio Inc.
- Takeda Pharmaceutical Company Limited
- Thermo Fisher Scientific Inc.
- Vericel Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
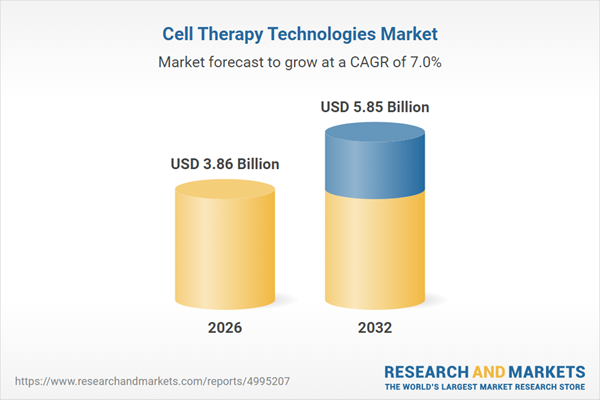
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 3.86 Billion |
| Forecasted Market Value ( USD | $ 5.85 Billion |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |