The COVID-19 pandemic disrupted the research and development activities of other therapies and drugs for medical conditions other than COVID-19. It impacted the treatment procedures and supply chains of pharmaceuticals and medical devices worldwide and the market for posterior segment eye disorders. For instance, according to a health article published in March 2021 by Oftalmologàa Barraquer, COVID-19 caused severe and irreversible consequences for people with glaucoma who suffered a very rapid evolution of the disease and have not been able to carry out follow-ups by the specialist, which, in some cases, resulted in a loss of the visual field. Therefore, the overall impact of COVID-19 on the posterior segment eye disorders market was adverse, primarily due to the decline in the diagnostics and treatment procedures of the diseases associated with the posterior segment of the eye. However, the rise in eye disorder surgeries worldwide to clear the backlogs of surgical procedures caused by the pandemic-related restrictions compensated for the market's growth in the later phase of the pandemic.
Posterior segment eye disorders are one of the major causes of visual impairments worldwide. Their prevalence is increasing gradually due to the increase in the prevalence of eye diseases, diabetes, and geriatric populations that are more vulnerable to eye ailments. With the increase in the burden of these diseases, the demand for diagnostics and treatment is increasing worldwide, driving the growth of the studied market.
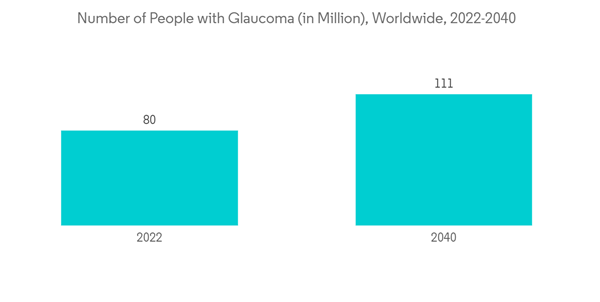
According to the World Glaucoma Association (WGA) 2022 update, around 79.6 million people were living with glaucoma in 2020, and the number is projected to reach 111.8 million people by 2040. It also highlighted that at least 50% of glaucoma sufferers are unaware of their condition, and around 90% of glaucoma cases in some developing countries are undetected. Thus, the surge in glaucoma is ultimately projected to boost the posterior segment eye disorders market over the forecast period.
With the launch of new products, research and development, collaboration, mergers, and acquisitions, the market for posterior segment eye disorders is expected to grow during the forecast period. For instance, in June 2022, Iridex Corporation received regulatory approval to market and sell its Cyclo G6 platform for the treatment of glaucoma diseases in China from its National Medical Products Administration (NMPA).
Due to the rising awareness about eye disorders and the factors mentioned above, the posterior segment eye disorders market is expected to register healthy growth over the forecast period. However, factors such as stringent regulatory policies of different countries and the lack of proper healthcare infrastructure in developing and under-developing countries are likely to restrain this growth.
Posterior Segment Eye Disorders Market Trends
Small Molecules by Drugs Segment is Expected to Hold a Significant Market Share Over the Forecast Period
Compounds with a low molecular weight that can change the biochemical processes for treating diseases are known as small-molecule drugs. Factors such as the growing burden of eye disorders, increasing R&D for the innovation of new therapeutics, and the launch of products are driving segmental growth.As per the study published in November 2021 by the National Library of Medicine, around 103.12 million adults were living with diabetic retinopathy globally in 2020, and the number is projected to increase to 160.50 million by 2045. Thus, the growing burden of eye disorders is likely to increase the demand for their treatment, thus driving the segment.
The market segment is also boosted by increasing clinical studies and collaboration among the market players. For instance, in December 2021, AbbVie Inc. (Allergan) received approval for VUITY (pilocarpine HCl ophthalmic solution) 1.25% by the US FDA to treat presbyopia and be available by prescription in pharmacies in the United States. Such launches are expected to drive the growth of the market segment. Thus, the small molecules segment is expected to project growth over the forecast period.
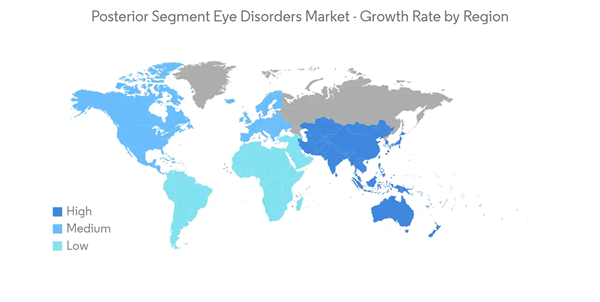
North America is Expected to Hold a Major Market Share Over the Forecast Period
North America is anticipated to have significant market growth owing to its well-established healthcare infrastructure, the presence of key market players, new product launches, and the rising burden of posterior eye disorders in the region.The growing mergers and acquisitions are one of the key reasons for the market's growth. For instance, in November 2021, Alcon acquired Ivantis Inc. to expand its product portfolio by adding the Hydrus micro stent for surgical glaucoma. Hydrus micro stent is one of the key products in Canada. With this acquisition, Alcon expanded its presence in the market.
In September 2021, Zilia Inc. received USD 3.16 million through seed financing. This would aid the company's entry into ocular diagnostics by increasing the number of posterior segment eye disorders diagnoses. The product can measure oxygen saturation in the eye, an important biomarker for eye diseases such as glaucoma, diabetic retinopathy, and age-related macular degeneration.
Hence, owing to such factors, the North American market is expected to have faster growth over the forecast period.
Posterior Segment Eye Disorders Industry Overview
The market for posterior segment eye disorders is moderately fragmented. Market players are focusing on new product launches, product innovation, regional expansions, and collaborations to increase their market share. The key market players operating in the market include F. Hoffmann-La Roche AG, Regeneron Pharmaceuticals Inc., Rainbow Medical Ltd (Nano Retina), Second Sight Medical, and Merck & Co. Inc.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alcon Inc.
- Abbvie Inc. (Allergen PLC)
- Bausch Health Companies Inc.
- F Hoffmann-La Roche
- Merck & Co. Inc.
- Novartis AG
- Santen Pharmaceuticals
- Rainbow Medical Ltd (Nano Retina)
- Regeneron Pharmaceuticals Inc.
- Second Sight Medical Products Inc.
- Aerie Pharmaceuticals