The emergence of the COVID-19 pandemic has impacted the United Kingdom's in-vitro diagnostics market. For instance, an article published by the AMS in September 2021, stated the urgent requirement to diagnose increasing COVID-19-positive patients accelerated the development of IVD kits across the UK. Thus, COVID-19 surged the demand for IVD kits and the market witnessed significant growth during the pandemic. However, in the current scenario, with the decrease in COVID-19 cases, the demand for IVD may get stabilized compared to the initial days of the pandemic but the presence of other diseases may keep on increasing the demand for IVD kits over the forecast period.
The major factors fueling the market growth are the rising prevalence of chronic disease increased demand for early and effective diagnostic tests, increased use of point-of-care (POC) diagnostics, and increasing awareness and acceptance of personalized medicine and companion diagnostics have also helped the market growth.
The rising prevalence of chronic diseases is a major factor driving market growth. For instance, the BHF published CVD factsheet in Febrauray 2023, which reported there were about 7.6 million people in the UK living with heart and circulatory diseases, of which 4 million people were male, and 3.6 million were female in the year 2021. Similarly, diabetes is one of the most prevalent chronic diseases in the UK. For instance, in 2021, the IDF reported that 6.3% of individuals in the country aged between 20 years to 79 years were living with diabetes and this number is projected to increase by 7.5% by 2045. Thus, a high number of people living with heart and circulatory diseases and diabetes are driving the demand for in-vitro diagnostics thus fueling the growth of the studied market.
Also, the rising geriatric population of the country will have a positive outlook on the market, as it is more prone to diseases and other medical conditions. For instance, a report published United Kingdom Office of National Statistics (ONS) in November 2022, stated that over 11 million people in England and Wales account for 18.6% of the total population aged 65 years or older, compared with 16.4% at the time of the previous census. This included over 527,900 people who were at least 90 years of age. Since older people are more prevalent to heart diseases and other chronic diseases, the increasing geriatric population is increasing the demand for IVDs, thereby driving the growth of the studied market.
Moreover, the introduction of point-of-care diagnostics and its increasing use is driving the growth of the studied market. For instance, in May 2022, LumiraDx, a UK-based healthcare company that manufactures a diagnostic platform to support a menu of tests reported that its HbA1c test received a CE mark for the screening and monitoring of people with diabetes in the point of care (POC) setting. Thus, the launch of such POC is also contributing to the growth of the studied market.
Furthermore, government initiatives in the country are expected to boost the growth of the market studied, during the forecast period. For instance, in November 2021, the UK Health Security Agency (UKHSA) Immunisation and Vaccine Preventable Diseases Division launched a new rash-fever surveillance scheme. This program aims to increase the number of samples tested for measles and rubella diagnostics testing, the program was initiated by the UK government and distributed rubella diagnostic kits for rapid rubella testing. Thus, such initiatives taken by the government to minimize the transmission of infectious diseases by increasing the sample diagnosis are driving the growth of the studied market.
Thus, the rising prevalence of chronic disease increased demand for early and effective diagnostic tests, increased use of point-of-care (POC) diagnostics, and increased awareness and acceptance of personalized medicine and companion diagnostics are driving the growth of the studied market. However, stringent regulatory frameworks in the country are expected to impede the growth of the in-vitro diagnostics market in the UK.
UK In-Vitro Diagnostics (IVD) Market Trends
Infectious Diseases Segment is Expected to Witness Significant Growth Over the Forecast Period.
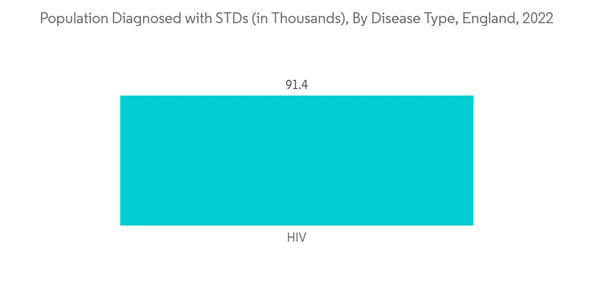
The infectious diseases segment is expected to hold a significant market share. Infectious diseases include HIV/AIDS, tuberculosis, hepatitis B, and hepatitis C, still posing a significant burden in the UK. For instance, in April 2022, the WHO reported 10 cases of severe acute hepatitis of unknown aetiology in children under 10 years, across central Scotland. By 8 April 2022, 74 cases had been identified in the UK. The source also reported that the UK has reported a recent unexpected significant increase in cases of severe acute hepatitis of unknown aetiology in young children. Thus, increasing cases of infectious diseases like hepatitis are driving the demand for IVD kits and thus driving the growth of the studied market.Moreover growing awareness regarding the early diagnosis and treatment, especially for infectious diseases in the country, is expected to contribute to market growth over the period. TB Alert is the UK's national tuberculosis charity raising public and professional awareness about TB. For instance, in July 2021, the UKHSA initiated the TB Action Plan for England, from 2021 to 2026, which aims to improve the prevention, detection, and control of TB in England. Thus, such initiatives are increasing the demand for IVD kits and contributing to the market's growth.
Hence, owing to the rising burden of infectious diseases, along with the expansion of the international market, players are driving the market growth for in-vitro diagnostics for infectious diseases in the UK.
Molecular Diagnostics Segment is Expected to Witness High Growth Over the Forecast Period.
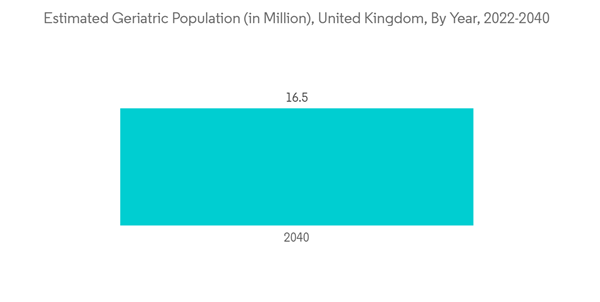
The molecular diagnostics segment is expected to witness significant growth over the forecast period owing to an increase in the geriatric population, the high prevalence of diseases like cancer, cardiovascular diseases, neurological disorders, diabetes, and obesity which could be linked to the rising geriatric population is driving the growth of this segment in the country. Also, government initiatives are leading to the development of better diagnostics in the country and are contributing to the growth of this segment.In July 2022, the UN population projections reported that in the year 2022, 12.9 million people aged 65 years and above. The number is anticipated to rise, even more, reaching 15.03 million in 2030 and 16.5 million in 2040. With the increasing burden of the geriatric population in the country, the burden of chronic diseases in the UK is also expected to rise, which, in turn, is anticipated to drive the demand for molecular diagnostic tests for diagnosing chronic diseases, thereby driving the growth of this segment.
Moreover, the technological developments leading to the development of the new product, are also fueling the growth of this segment. For instance, in October 2022, Thermo Fisher Scientific launched the TaqPath Enteric Bacterial Select Panel, a CE-IVD marked panel to detect common gastrointestinal bacteria in about two hours. The test enables clinicians to identify the root cause of an infection and administer the most appropriate treatment to their patients more quickly. Thus, such product launches are driving the growth of this segment.
Furthermore, government initiatives are also contributing to the growth of this segment. For instance, in June 2022 MHRA published a report focussing on the development of IVDs and medical devices in the UK. The report also stated that government to protect patients and the public and facilitate access for UK patients, the UK government has initiated the regulation of in vitro diagnostics and increased its production. Thus, such initiatives are contributing to the growth of this segment.
UK In-Vitro Diagnostics (IVD) Industry Overview
United Kingdom's in vitro diagnostics market is highly competitive. The market consists of several major players operating globally and regionally. Some of the companies working in this field are Abbott Laboratories, Becton, Dickinson, and Company, BioMerieux SA, Bio-Rad Laboratories, Inc., Danaher Corporation, F. Hoffmann-La Roche AG, Qiagen N.V., Siemens Healthineers AG, Sysmex Corporation, Thermo Fischer Scientific Inc., and Fujifilm Holdings Corporation.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.