The COVID-19 pandemic had a significant positive impact on the market studied. Globally, the risk of cardiovascular diseases (CVDs) among the target population increased during the pandemic. For instance, a research article published in JAMA in March 2022 stated that when the large pool study was conducted among COVID-19 patients from March 2020 to January 2021, various cardiovascular diseases, such as cerebrovascular disorders, dysrhythmias, ischemic and non-ischemic heart disease, pericarditis, myocarditis, heart failure, and thromboembolic illness, were frequently found in COVID-19 patients. Thus, the demand for cardiac diagnosis and the use of cardiac markers for diagnostic purposes increased during the pandemic. However, currently, the market has reached its pre-pandemic nature and is expected to witness strong growth in the coming years.
The rising prevalence of CVDs globally and the growing emphasis on preventing misdiagnoses of cardiac conditions are the major factors that contribute to the market's growth. For instance, as per a press release by Boehringer Ingelheim GmbH in August 2021, nearly 60 million people or more were affected by the chronic, disabling cardio-renal-metabolic illness known as heart failure across the world every year. In addition, the June 2021 update by WHO showed that the burden of CVD rose globally, especially in low- and middle-income countries. Thus, to control the disease burden, a better diagnosis is required. This creates a demand for cardiac markers, which necessitates the availability of advanced devices capable of analyzing the cause or risk factors of CVD and associated diseases. As a result, the global demand for cardiac marker analyzers is increasing, which is expected to propel market growth.
Furthermore, the increasing number of hospital admissions and critical care unit admissions due to CVD creates a demand for the availability of cardiac markers for better disease diagnosis. For instance, as per data released by the AIHW in May 2022, out of 11.8 million hospital admissions, 7% involved a stay in intensive care nits, and 3.8% involved emergency room admissions. This high number of admissions of CVD patients in emergency and critical care creates the need for the development and availability of cardiac marker analyzers that can read the markers and result in a better diagnosis of the disease. This is expected to drive the growth of the market studied.
Therefore, due tfactors mentioned aboveactors, the market studied is expected to grow significantly during the forecast period. However, a strict regulatory process and the high cost of the instruments are expected to restrain the market growth during the study period.
Cardiac Marker Analyzer Market Trends
Immuno-Fluorescence Analyzers (IFA) Segment is Expected to Hold the Largest Share in the Market
The immunofluorescence analyzers (IFA) segment is expected to account for the highest cardiac marker analyzer market share. Compared to other analyzers, their dominance in the revenue share can be attributed to their use as the gold standard for the early diagnosis of cardiac diseases. In addition, owing to their ability to quantify the concentration of biomarkers in whole blood, serum, and plasma to assist clinical diagnosis, there is a growing demand for IFA analyzers. Thus, the segment is expected to witness growth.Among all major cardiovascular disorders, myocardial infarction (MI) has a high morbidity and mortality rate. Thus, it is crucial to identify the right biomarkers as soon as possible to prevent the occurrence of MI and other serious outcomes. Various types of biomarkers can be tested using IFA analyzers, including the cardiac markers Troponin I, NT-proBNP, h-FABP, CK-MB, myoglobin, and others.
According to an article published in PMC in August 2021, cardiac troponin, a crucial component of muscle control and contraction, was one of the most specific biomarkers for cardiac damage because of its high specificity. The same source stated that numerous diagnostic techniques have been developed, but IFA was discovered to be a quick, precise, and sensitive instrument for determining cardiac troponins post-MI. Thus, such research is fueling the segment's growth. Therefore, owing to the abovementioned factors, the segment is expected to grow significantly during the forecast period.
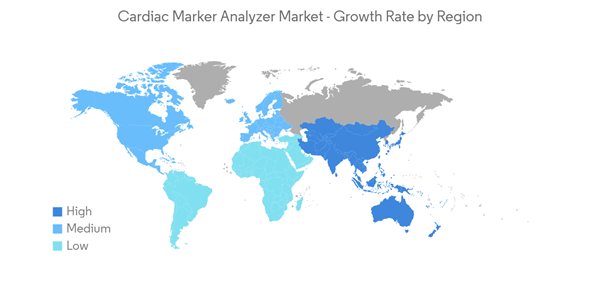
North America is Expected to Have a Significant Growth Over the Forecast Period
North America is expected to dominate the overall cardiac marker analyzer market throughout the forecast period. The major factors attributing to the growth are a sedentary lifestyle, a lack of physical activity, a lack of a proper diet, anxiety, and stress, resulting in the growing prevalence of multiple cardiovascular diseases like coronary artery disease, strokes, and atrial fibrillation. The United States contributes significantly to the market's growth among other regional countries.Various sources and research state that troponin is often used as a cardiac biomarker to diagnose and risk stratification patients with suspected acute coronary syndrome (ACS). When the heart muscle has been damaged, like in the case of a heart attack, these troponin I or T proteins are released. The blood's troponin I or T levels are determined using a troponin test with immunoassay analyzers. Thus, with an increase in cardiac patients in countries across North America, the demand for cardiac markers is increasing, which is expected to fuel the market's growth. For instance, the January 2022 update from Cedars-Sinai showed that coronary artery bypass graft surgery (CABG), also known as coronary artery bypass or bypass surgery, is the most common type of heart surgery, and more than 300,000 people have successful bypass surgery in the United States each year.
In addition, the July 2022 update by the CDC showed that coronary heart disease was the most common type of heart disease, and approximately 20.1 million adults aged 20 and older had the disease in the United States. Additionally, per the source above, every 40 seconds, someone suffers from a heart attack, and nearly 805,000 people in the United States have a heart attack every year. Thus, the high burden of cardiovascular diseases demands the availability of advanced diagnostic options for better treatment, thereby driving the demand for cardiac markers. This is expected to drive the demand for cardiac marker analyzers, fueling market growth in the region.
Hence, due to the abovementioned factors, the market studied is expected to witness significant growth over the forecast period in North America.
Cardiac Marker Analyzer Industry Overview
The cardiac marker analyzer market is competitive and has several major players globally. The key players are developing novel products to compete with the existing products, while others are acquiring and partnering with the other companies trending in the market to expand their global presence. Some key players operating in the market are F. Hoffmann-La Roche Ltd., Abbott Laboratories, Quidel Corporation, CardioGenics Holdings Inc., and LSI Medience Corporation.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- F. Hoffmann-La Roche Ltd
- Quidel Corporation
- CardioGenics Holdings Inc.
- Abbott Laboratories
- PHC Holdings Corporation (LSI Medience Corporation)
- Thermo Fisher Scientific (Fisher Scientific)
- Siemens Healthineers AG
- Danaher (Beckman Coulter Inc.)
- Trinity Biotech PLC
- Boditech Med Inc.
- Agappe Diagnostics Ltd
- Hangzhou Laihe Biotech Co. Ltd