Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these positive drivers, the market encounters a substantial obstacle regarding the high price of testing and variable reimbursement policies. This financial hurdle limits access for the general public, especially within developing nations and lower-income demographics, thereby preventing NIPT from becoming a universally implemented standard in routine prenatal care. Consequently, these economic barriers impede the global market's ability to reach full saturation.
Market Drivers
Strategic partnerships and collaborations among leading market players are essential for broadening the global reach and capabilities of diagnostic providers in the NIPT sector. By acquiring regional laboratories and forming alliances, major companies are consolidating the market to strengthen distribution networks and incorporate specialized testing technologies into their portfolios. This consolidation facilitates the efficient delivery of prenatal screening services to diverse patient populations and accelerates the availability of comprehensive women's health solutions. For example, Labcorp announced in its 'Fourth Quarter and Full Year 2024 Results' in February 2025 that it completed 10 transactions throughout 2024 to solidify its position as a trusted partner to health systems, significantly reinforcing its presence in high-growth areas such as women's health.The rising average maternal age globally serves as a primary catalyst for the sustained demand for chromosomal aneuploidy screening. As delayed childbearing trends continue due to changing socio-economic factors, the increased clinical risk of chromosomal abnormalities in older mothers necessitates reliable, non-invasive fetal health evaluations. According to the CDC's 'Births: Provisional Data for 2024' report released in April 2025, the birth rate for women aged 40-44 rose by 2% in 2024, highlighting this significant demographic shift. This increase in higher-risk pregnancies correlates directly with higher utilization rates of prenatal diagnostics; Myriad Genetics reported in February 2025 that prenatal testing revenue grew 12% year-over-year, reflecting the strong uptake of these essential screenings driven by demographic pressures.
Market Challenges
The Global Non-invasive Prenatal Testing (NIPT) Market is significantly constrained by high testing costs and the absence of consistent reimbursement policies across various regions and insurance providers. Although NIPT is acknowledged for its accuracy, substantial out-of-pocket expenses create a severe barrier to entry for a large segment of the population, particularly in developing economies and among average-risk pregnancies where insurance coverage is frequently denied or limited. This financial disparity prevents the test from becoming a universal standard of care, effectively categorizing it as a premium service rather than a routine medical procedure available to all expectant mothers.As a result, market penetration remains uneven, with growth heavily concentrated in demographics that benefit from full financial coverage. In 2025, the German Medical Association highlighted this discrepancy, revealing that only approximately 25% of pregnant women under the age of 26 accessed NIPT services due to funding limitations, compared to significantly higher adoption rates in age groups with established reimbursement coverage. This divide illustrates how economic factors and inconsistent payer policies directly restrict the scalable expansion of the NIPT market, limiting its potential to reach saturation in the broader prenatal care sector.
Market Trends
The expansion of testing coverage to include average-risk pregnancies marks a fundamental shift in the market, transitioning the consumer base from exclusively high-risk cases to the broader general population. This evolution differs from demographic drivers as it relies on the normalization of screening as a standard routine for all expectant mothers regardless of age, supported by increasing clinical validation for younger demographics. The uptake in this wider segment is rapidly accelerating test volumes, creating a new primary engine for industry expansion. Evidencing this mass-market adoption, Natera reported in its 'Third Quarter 2025 Financial Results' in November 2025 that the company processed approximately 893,600 tests, a 15.2% increase year-over-year, driven largely by the robust penetration of these screenings into broader patient categories.Concurrently, the industry is advancing through the development of comprehensive multi-gene panel testing, which extends diagnostic capabilities beyond standard aneuploidy detection. Manufacturers are innovating assays that identify specific single-gene disorders and rare conditions previously undetectable via non-invasive methods, thereby addressing the clinical need for higher diagnostic yield in early pregnancy. This technological evolution enables the screening of complex inherited conditions, providing deeper genetic insights that facilitate earlier interventions. Highlighting this advancement, Natera announced in October 2025 the expansion of its single-gene testing panel to cover 20 genes, significantly enhancing its capability to detect challenging homozygous mutations and severe early-onset disorders.
Key Players Profiled in the Non-invasive Prenatal Testing (NIPT) Market
- YOURGENE HEALTH PLC
- Illumina, Inc.
- Natera, Inc.
- F.Hoffman La Roche Ltd.
- Perkin Elmer Inc.
- Laboratory Corporation of America Holdings
- Eurofins LifeCodex GmbH
- Progenity, Inc.
- Genesis Genetics
- Quest Diagnostics Incorporated
Report Scope
In this report, the Global Non-invasive Prenatal Testing (NIPT) Market has been segmented into the following categories:Non-invasive Prenatal Testing (NIPT) Market, by Product Type:
- Consumables
- Instruments
Non-invasive Prenatal Testing (NIPT) Market, by Test Type:
- Materni 21
- Harmony
- Panaroma
- Verifi
- NIFTY
- Others
Non-invasive Prenatal Testing (NIPT) Market, by Method:
- Ultrasound Detection
- Biochemical Screening Testing
- Cell-Free DNA Maternal Plasma Tests
- Fetal Cells in Maternal Blood Tests
- Others
Non-invasive Prenatal Testing (NIPT) Market, by Application:
- Trisomy
- Microdeletion Syndrome
- Others
Non-invasive Prenatal Testing (NIPT) Market, by End User:
- Diagnostic Laboratories
- Hospitals
- Others
Non-invasive Prenatal Testing (NIPT) Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Non-invasive Prenatal Testing (NIPT) Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Non-invasive Prenatal Testing (NIPT) market report include:- YOURGENE HEALTH PLC
- Illumina, Inc.
- Natera, Inc.
- F.Hoffman La Roche Ltd.
- Perkin Elmer Inc.
- Laboratory Corporation of America Holdings
- Eurofins LifeCodex GmbH
- Progenity, Inc.
- Genesis Genetics
- Quest Diagnostics Incorporated
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
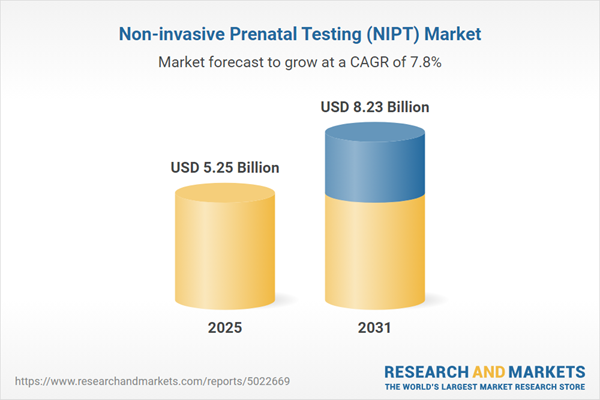
| Estimated Market Value ( USD | $ 5.25 Billion |
| Forecasted Market Value ( USD | $ 8.23 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |