Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
In this expansive market, numerous pharmaceutical companies, suppliers, and distributors collaborate to ensure the production and seamless supply chain of these essential medicinal ingredients. The continuous advancements in research and development efforts contribute to the relentless pursuit of improving the efficacy and safety profiles of immunosuppressants. This ongoing progress is vital in meeting the increasing demand driven by the growing prevalence of transplant procedures and autoimmune conditions worldwide.
For instance, in May 2023, Millipore Sigma announced a USD 69 million expansion of its U.S. facility, aiming to double its manufacturing capacity for highly potent active pharmaceutical ingredients (HPAPIs). The expanded facility will support the development and commercial production of antibody drug conjugates (ADCs), enhancing the company’s capabilities in targeted cancer therapies and reinforcing its position in the growing market for specialized pharmaceutical manufacturing. The Global Immunosuppressants API Market is characterized by its global scope, with diverse stakeholders working together to address the evolving healthcare needs of patients. From innovative drug formulations to optimized manufacturing processes, the industry strives to deliver cutting-edge solutions to healthcare professionals and patients alike. As the understanding of immune system regulation deepens and technology continues to evolve, this market holds immense potential for further growth and innovation.
Key Market Drivers
Rising Prevalence of Autoimmune Diseases
The rising prevalence of autoimmune diseases represents a significant healthcare challenge worldwide. Autoimmune disorders, such as rheumatoid arthritis, lupus, and multiple sclerosis, occur when the immune system mistakenly attacks healthy cells and tissues. As these conditions become more prevalent, there is a growing need for effective treatments, and this has led to an increase in the demand for immunosuppressants API (Active Pharmaceutical Ingredients). For instance, according to the Alzheimer’s Association, over 6 million people in the U.S. were affected by Alzheimer’s disease in 2023, making it the sixth leading cause of death, primarily impacting those aged 65 and older.This number is projected to reach 12.7 million by 2050, significantly increasing the demand for drugs and microbial APIs related to Alzheimer’s and similar neurodegenerative conditions, driving growth in pharmaceutical development and manufacturing. Healthcare awareness and diagnostics improve, more individuals are being correctly diagnosed with autoimmune diseases, leading to an increased demand for treatment options. Secondly, as life expectancy increases, the aging population is more susceptible to autoimmune conditions, further fueling the need for immunosuppressant medications. Thirdly, ongoing research and development efforts are leading to the discovery of newer and more targeted immunosuppressant drugs, which, in turn, are stimulating market growth.
The rising prevalence of autoimmune diseases is expected to drive significant growth in the immunosuppressants API market. This growth is not only beneficial for pharmaceutical companies but also for the countless individuals suffering from autoimmune disorders, as it promises better access to innovative and effective treatments that can help them manage their conditions and lead healthier lives.
Key Market Challenges
High Costs Associated With API
The high costs associated with Active Pharmaceutical Ingredients (APIs) in the immunosuppressants market pose a significant challenge to the growth of this sector. The complex and labor-intensive processes involved in manufacturing immunosuppressant APIs, along with the stringent quality control requirements and regulatory compliance, contribute to the elevated costs of production. The research and development phase for immunosuppressant drugs demands substantial investments in terms of time and resources. Identifying and synthesizing effective compounds, conducting preclinical and clinical trials, and meeting regulatory requirements can lead to substantial expenses. These costs are often passed on to consumers and healthcare systems, which can result in limited access to these vital medications, especially in lower-income regions.The manufacturing of immunosuppressant apis requires specialized facilities, equipment, and highly skilled personnel to ensure product consistency and quality. Maintaining these facilities and adhering to Good Manufacturing Practices (GMP) standards further escalates production costs. These expenditures, again, contribute to the high price tags of immunosuppressant drugs, making them less affordable for many patients.
The ongoing research to develop more targeted and efficient immunosuppressants often involves the exploration of cutting-edge technologies and innovative drug delivery systems. While these advancements can lead to improved therapeutic outcomes, they can also add to the overall cost burden of drug production.
The high costs associated with immunosuppressant apis have the potential to impede their growth and accessibility. Efforts to mitigate these challenges, such as increasing efficiency in manufacturing processes and promoting competition in the pharmaceutical industry, are essential to ensure that individuals who require immunosuppressant therapies can access them without financial hardship, ultimately improving the overall healthcare landscape.
Key Market Trends
Expansion Of Healthcare Infrastructure
The expansion of healthcare infrastructure is expected to play a pivotal role in driving the growth of the Immunosuppressants API (Active Pharmaceutical Ingredients) market in the future. A robust healthcare infrastructure is essential for efficiently diagnosing, treating, and managing various medical conditions, including autoimmune diseases and organ transplantations, where immunosuppressant drugs are instrumental. An expanded healthcare infrastructure means increased access to medical facilities and specialized clinics. This facilitates earlier diagnosis and treatment of autoimmune diseases, leading to a larger patient pool requiring immunosuppressant therapies. The establishment of well-equipped transplant centers and surgical facilities in various regions enhances the capacity to perform organ transplantations, leading to higher demand for immunosuppressant medications in both developed and developing countries.The availability of state-of-the-art healthcare facilities supports the adoption of cutting-edge technologies and treatments. This includes advanced diagnostic tools, precision medicine approaches, and innovative immunosuppressant therapies that offer better patient outcomes and fewer side effects. As the healthcare infrastructure continues to grow, it encourages research and development efforts aimed at improving immunosuppressant drugs, further boosting market growth. A robust healthcare system often involves better health insurance coverage and government support for patient care. This can alleviate the financial burden on individuals requiring long-term immunosuppressant treatments, making these medications more accessible and affordable. Government policies that prioritize the expansion of healthcare infrastructure can facilitate increased investment in pharmaceutical research and production, driving innovation and competition in the immunosuppressants API market.
Key Market Players
- Biocon Ltd.
- NATCO Pharma Limited
- Triveni Interchem Private Limited
- Concord Biotech Ltd.
- RPG Life Sciences Limited
- Jiangsu Jiuyang Bio-Pharmaceutical Co. Ltd.
- Chunghwa Chemical Synthesis & Biotech Co. Ltd.
- AbbVie Inc.
- Pfizer Inc.
- Zhejiang Hisun Pharmaceutical Co. Ltd.
Report Scope:
In this report, the Global Immunosuppressants API Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Immunosuppressants API Market, By Method:
- Synthetic Chemistry
- Peptide chemistry
- Fermentation
- Chromatographic purification
Immunosuppressants API Market, By Application:
- Autoimmune Disease
- Organ Transplant
Immunosuppressants API Market, By Type:
- Corticosteroids
- Janus Kinase Inhibitor
- Calcineurin Inhibitors
- mTOR Inhibitor
- Others
Immunosuppressants API Market, By Product:
- Tablets
- Capsules
- Liquids
- Injections
Immunosuppressants API Market, By APIs:
- Tacrolimus
- Sirolimus
- Everolimus
- Mycophenolate Mofetil
- Mycophenolate Sodium
Immunosuppressants API Market, By End User:
- Biotechnology & Biopharmaceutical Companies
- CMOs
- CROs
- CDMOs
- Others
Immunosuppressants API Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Immunosuppressants API Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Biocon Ltd.
- NATCO Pharma Limited
- Triveni Interchem Private Limited
- Concord Biotech Ltd.
- RPG Life Sciences Limited
- Jiangsu Jiuyang Bio-Pharmaceutical Co. Ltd.
- Chunghwa Chemical Synthesis & Biotech Co. Ltd.
- AbbVie Inc.
- Pfizer Inc.
- Zhejiang Hisun Pharmaceutical Co. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | August 2025 |
| Forecast Period | 2024 - 2030 |
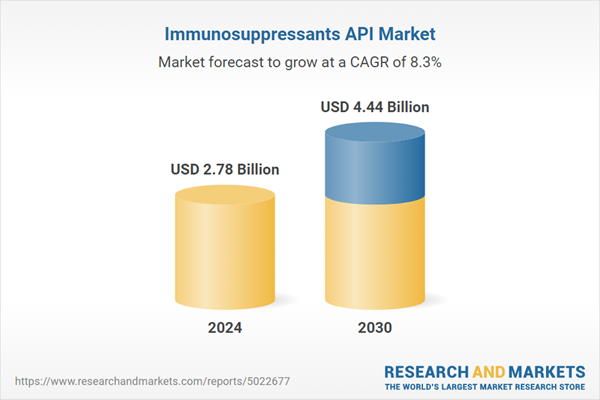
| Estimated Market Value ( USD | $ 2.78 Billion |
| Forecasted Market Value ( USD | $ 4.44 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |