COVID-19 had significantly affected the endoscope reprocessing market owing to the abrupt shutdown of reprocessing facilities in the initial days of the pandemic due to the strict lockdown regulations. Also, the reductions in endoscopy procedures during the initial stages of the pandemic had a notable impact on the growth of the market. However, the adoption of the appropriate measure to follow the endoscope reprocessing procedures during the post-pandemic period is expected to drive the growth of the market. For instance, the NCBI article published in October 2021, mentioned that endoscope reprocessing staff should continue to adopt appropriate personal protective equipment including gloves, gowns, face shields, and other equipment are recommended. Such guidelines for the safe reprocessing of endoscopes are expected to drive the growth of the market over the forecast period.
The major factors driving the growth of the endoscope reprocessing market include the rising cases of endoscopy-related infections, growing hospital investments in endoscopy instruments, and the rising number of diseases that require endoscopy procedures. Increased usage of endoscopic devices has been observed in disease diagnosis and treatment. The growing burden of respiratory diseases, such as lung cancer, and chronic obstructive pulmonary disorders is propelling the market's growth over the forecast period. For instance, according to the NCBI article published in March 2022, the estimated pooled prevalence of COPD was 11.1% in South Asian countries in 2021. The report also mentioned that the prevalence of COPD was highest in North India (19.4%) and Bangladesh (13.5%). Such a high burden of Chronic Obstructive Pulmonary Disease is expected to drive the demand for endoscopy procedures, thereby contributing to the growth of the market.
Additionally, the rising number of cancer cases is also expected to contribute to the demand for endoscopy reprocessing as endoscopy procedures are widely used in diagnosing cancer, thereby contributing to the market's growth. For instance, IARC 2021 reported that out of these cases, 1.15 million cases are of colon cancer, which is expected to reach 1.92 million by 2040. Also, the NCBI article published in July 2021, mentioned that the age-standardized incidence rates of colorectal cancer in India were 7.2 and 5.1 per 100,000 population for men and women. Such a rapidly rising burden of different types of cancers, in which endoscopes are largely used for diagnostic purposes, is boosting the growth of endoscope reprocessing over time.
Furthermore, rising demand for surgeries with faster recovery, fewer post-surgery infections, less pain, reduced scarring, better control of bleeding, and increased accuracy are anticipated to boost the demand for various endoscopy devices. Major players in the market are focusing on the approval of new products to expand their product portfolio. For instance, in April 2021, Advanced Sterilization Products received the United States Food and Drug Administration’s support for the ASP AEROFLEX Automatic Endoscope Reprocessor. Thus, the rising number of endoscopy reprocessing devices in the market will likely lead to high growth of the market.
However, the dearth of skilled professionals to carry out endoscopic procedures and the concerns regarding the safety of reprocessed instruments are the factors expected to hinder the market growth.
Endoscope Reprocessing Market Trends
Automated Endoscope Reprocessors Segment is Expected to Register Good Growth Over the Forecast Period
Automated endoscope reprocessors are designed to kill microorganisms in or on reusable endoscopes by exposing their outside surfaces and interior channels to high-level disinfectant or liquid chemical sterilant solutions. Also, the suggested usage of automated endoscope reprocessors in various guidelines is expected to contribute to the growth of the studied segment. For instance, in January 2021, the Single Use Endoscopy article guidelines enlisted the GI societies' updated guidelines which mentioned that under normal circumstances, one cycle of high-level disinfection, preferably in an automated endoscope reprocessor is enough to ensure adequate reprocessing.Likewise, the increasing product launches and approvals, given the added features of automated endoscope reprocessors, are expected to drive the studied segment over the forecast period. Similarly, in June 2022, Getinge launched an updated version of the ED-Flow automated endoscope reprocessors. ED-Flow efficiently performs leak testing, cleaning, and high-level disinfection of flexible endoscopes, delivering effective, reliable results. New process indicator lights have been added to allow easy visibility of the process status from across the room, which creates improved department workflow leading to higher productivity. Hence, such innovative product launches would improve efficiency and will fuel market growth.
Additionally, in May 2021, the United States Food and Drug Administration (FDA) granted 510(k) clearance for Advanced Sterilization’s ASP Aeroflex, a device that automatically cleans and sterilizes endoscopes. The Advanced Sterilization device can automatically clean and disinfect flexible endoscopes from multiple manufacturers in 22 minutes, eliminating the need to use manual test strips to measure the disinfectant’s concentration. Hence, such approvals are likely to increase segment growth.
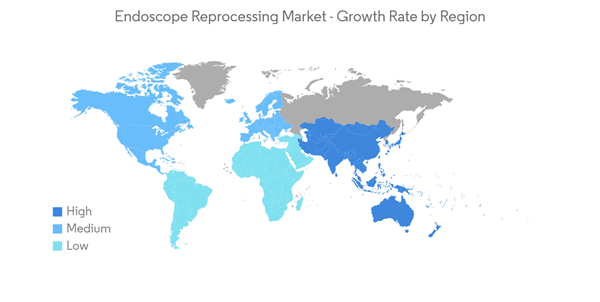
North America is Expected to Hold Significant Market Share Over the Forecast Period
North America holds the majority of the share and in the North American region, the United States holds the major share. The rising number of disorders and the increasing launches and acquisitions by key market players in the region are the major drivers for the growth of the studied market. Increasing cases of cancer in this region are also expected to drive the demand for endoscopy procedures, thereby fueling the growth of the market.For instance, according to the Canadian cancer society data 2022, nearly 24,300 Canadians will be diagnosed with colorectal cancer in 2022. Thus, increasing colorectal cancer would require a higher diagnostic rate, which will fuel the demand for endoscopy and hence will increase the growth rate of the studied market.
Additionally, the increasing number of product launches and high concentration of major players in the region is expected to boost the market. For instance, in January 2021, Olympus launched the endoscope storage cabinet, the ScopeLocker with HEPA-hinged door endoscope storage system. This line expansion of Olympus' infection prevention offering addresses a rapidly shifting environment in the gastrointestinal field. Also, in January 2021, IDESCO launched the endoscope drying storage cabinet called the IDESCO ENDO D3. Hence, all the above-mentioned factors are expected to augment the growth of the North American market over the forecast period.
Endoscope Reprocessing Market Competitor Analysis
The market studied involves both multinational and local companies owing to a large number of devices and consumables being used in the reprocessing process. Some of the market players are Advanced Sterilization Products (Johnson & Johnson Company), Cantel Medical, Custom Ultrasonics, Ecolab Inc, Endo-Technik W. Griesat, Getinge AB, Metrex Research, LLC., Olympus Corporation, STERIS plc., and Wassenburg Medical (A Member of Hoya).Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Cantel Medical
- Custom Ultrasonics
- Ecolab Inc
- Endo-Technik W. Griesat
- Getinge AB
- Envista Holdings Corporation (Metrex Research LLC)
- Olympus Corporation
- Fortive Corporation (Advanced Sterilization Products)
- STERIS plc.
- HOYA Corporation
- Steelco S.p.A.
- BES Rehab Ltd
- ARC Group of Companies Inc
- Shinva Medical Instrument Co. Ltd.