The COVID-19 pandemic presented significant hurdles for the healthcare sector. Since the majority of surgical procedures were considered to be non-urgent, all outpatient consultations were delayed or restricted during the COVID-19 pandemic to lower the danger of viral transmission. Thus, the lacrimal duct stent market was adversely impacted during the pandemic. However, it is expected to gain traction in the post-pandemic era due to the resumption of surgical procedures.
Certain factors that are driving the market include the increasing patient pool for nasolacrimal duct obstruction and rising demand for treatments with minimal invasions and better success rates. Nasolacrimal duct obstruction (NLDO) or dacryostenosis is the most common disorder of the lacrimal system. According to the August 2022 updated report, published by the NCBI, congenital nasolacrimal duct obstruction may occur in 3-6% of newborns, while it occurs bilaterally in 20% of newborns. There is a high rate of spontaneous resolution of congenital nasolacrimal duct obstruction, with approximately 70% of affected children being free of the symptoms by 3 months of age, and over 90% recovering by their first birthday. However, infants at increased risk for this condition include those with trisomy 21, ectrodactyly-ectodermal dysplasia-cleft lip/palate (EEC) syndrome, branchiooculofacial syndrome, CHARGE (coloboma, heart anomaly, choanal atresia, and retardation, genital, and ear anomalies) syndrome, and Goldenhar syndrome. Hence, the rising prevalence of such health conditions is expected to drive the market over the forecast period.
New studies on lacrimal stent tubes help expand the applications for various conditions, which is expected to boost the market's growth over the forecast period. For instance, as per the study published by the European Journal of Ophthalmology in December 2021, lacrijet monocanalicular stent intubation has a high rate of success, shortens the surgical time, and has a low rate of complications in children with congenital nasolacrimal duct obstruction. Hence, the new studies on lacrimal stent tubes expand the indications and provide the safety and efficacy of the stents, which may drive the market over the forecast period.
However, complications associated with stent tubes are likely to hinder the growth of the market over the forecast period.
Key Market Trends
Bicanalicular Lacrimal Duct Stent Tube is Expected to Hold Significant Share in the Market During the Forecast Period
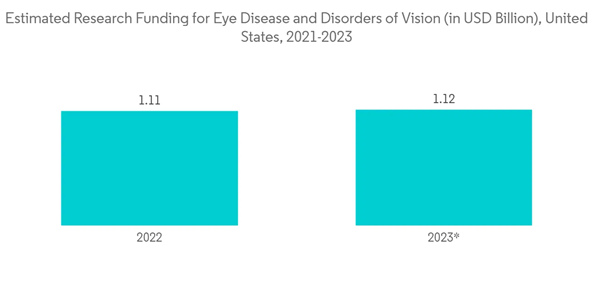
Bicanalicular lacrimal intubation is a successful treatment for children with nasolacrimal duct obstruction, and the younger the age of the children, the higher the success rate. Bicanalicular lacrimal tubes have two probes with an intervening stent. One probe is passed through the upper punctum and the other through the lower punctum. The probes are removed, and the free ends of the silicone tube are tied in the nose and sometimes secured with a suture. The longer the duration of the tube retention, the better the results, with the best results between 3 and 12 months. The increased research funding on eye disorders increases the scope and usage of the eye-related devices like bicanalicular lacrimal ducts stent tubes, which may drive the market over the forecast period.The studies showing the reduced complications of bicanalicular stents are boosting the usage of these stents. For instance, as per the report published by the Journal of Ophthalmology in March 2021, silicone tube repositioning guiding by using a memory wire probe is an optional technique in the treatment of prolapse of silicone tubes in bicanalicular nasal iintubation.
The comparative studies of bicanalicular lacrimal intubation versus monocanalicular lacrimal intubation help reveal the advantages of bicanalicular lacrimal intubation over monocanalicular lacrimal intubation, which is expected to drive the market's growth through this segment. For instance, as per the study published by Frontiers in July 2022, endoscopic dacryocystorhinostomy with pushed bicanalicular intubation is easily manipulated and has a promising surgical outcome over pulled monocanalicular intubation. Hence, the better outcomes of bicanalicular intubation using a stent tube are expected to drive the market over the forecast period.
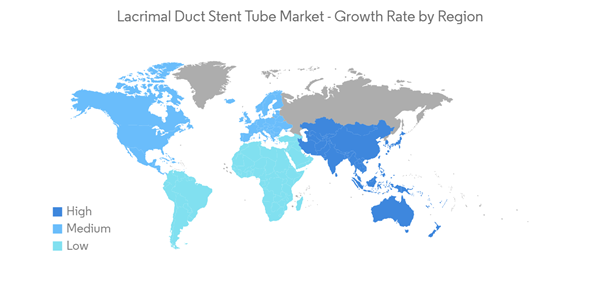
North America is Expected to Have Significant Growth in the Market Over the Forecast Period
North America is expected to have significant growth in the overall market throughout the forecast period. The United States holds the largest market share due to better regulations of surgical devices and growing awareness among the population to approach such procedures in case of lacrimal duct obstruction and other associated problems. The companies in the country also have well-established direct channel collaborations with hospitals and clinics.The high burden of nasolacrimal duct obstruction and new studies using lacrimal sten tubes in the region are boosting the demand for lacrimal stents in the region, thus driving the market in the region. As per the report published by the American Academy of Ophthalmology in November 2022, congenital nasolacrimal duct obstruction occurs in approximately 5% of normal newborn infants, and the blockage occurs most commonly at the valve of Hasner at the distal end of the duct. Hence, the high burden of congenital nasolacrimal duct obstruction increases the demand for a lacrimal stent for maintenance.
The new studies on lacrimal stents reveal the comparative efficacy of products that can impact the market's growth. For instance, as per the study report published by IOVS Journal in June 2021, both the Crawford and Nunchaku style lacrimal stents showed equivalent rates of improvement in epiphora during the short- and long-term follow-up visits. Hence, the stent studies help find out the efficacy of the products in the management of lacrimal disorders, which may also drive the market over the forecast period.
Competitive Landscape
The lacrimal duct stent tube market is consolidated and consists of very few major players. Companies like Aurolab, Bess Medizintechnik GmbH, ZEISS (FCI Ophthalmics), Gunther Weiss Scientific Glassblowing Co. Inc., and Kaneka Pharma America LLC hold a substantial market share.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aurolab
- Bess Medizintechnik GmbH
- ZEISS (FCI Ophthalmics)
- Gunther Weiss Scientific Glassblowing Co. Inc.
- Kaneka Medical America LLC
- BD (Becton, Dickinson and Company)
- BVI Medical Inc.
- Walsh Medical Devices Inc.
- Surtex Instruments Limited