Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Peptides serve as highly effective and selective signaling molecules, binding to specific cell surface receptors such as ion channels or G protein-coupled receptors (GPCRs), triggering intracellular reactions. Their outstanding pharmacological profile and intrinsic characteristics make peptides an ideal starting point for the development of new therapies. Notably, peptides demonstrate exceptional safety, tolerability, and efficacy in humans due to their specificity. When compared to protein-based biopharmaceuticals, peptide therapeutics exhibit lower processing complexity, resulting in production costs more akin to small molecules.
Key Market Drivers
Increasing Use in Pharmaceutical Industry
Peptides are highly potent and targeted pharmacological ingredients, exhibiting a diverse range of biological actions. Their broad chemical space, remarkable biological activity, and specificity, coupled with their relative simplicity of synthesis, ready availability, and low toxicity, position peptides as promising candidates for active medicinal components. Peptides can be designed to specifically target certain receptors, enzymes, or proteins in the body, allowing for highly targeted and precise therapeutic interventions. This targeted approach reduces the risk of off-target effects and enhances therapeutic efficacy. Peptides have a wide range of therapeutic applications. They can be used to treat various medical conditions, including cancer, metabolic disorders, cardiovascular diseases, autoimmune disorders, and infectious diseases.Peptides contribute significantly to the therapeutic landscape, particularly in the fields of oncology, diabetes, and obesity, generating billions of dollars in revenue. Moreover, the demand for peptides is steadily increasing for the treatment of renal failure, rare disorders, as well as cardiovascular and neurological conditions. Currently, there are over 100 peptide-based medications available, and with nearly 700 peptide medicines and therapeutic peptides in preclinical development, this number is projected to grow substantially.
Key Market Challenges
Purification and Quality Control
During peptide synthesis, side reactions and formation of by-products can occur, leading to impurities in the final product. Ensuring high purity involves careful optimization of the synthesis conditions to minimize the formation of impurities. Achieving high purity can be resource-intensive, as it may require substantial amounts of reagents, solvents, and specialized equipment. The cost of purification can add to the overall production expenses. Moving from small-scale to large-scale peptide synthesis can present challenges in maintaining consistent purity levels. Factors such as reaction kinetics and mass transfer can differ at larger scales, necessitating additional process optimization.Obtaining peptides of high purity is crucial for their successful application in research and therapeutics. Purification methods, such as high-performance liquid chromatography (HPLC), can be time-consuming and require optimization for each peptide sequence. Quality control measures are essential to ensure the accuracy, consistency, and safety of synthesized peptides. Maintaining high-quality standards and efficient purification processes can be a challenge for the peptide synthesis market.
Key Market Trends
Increasing Opportunity in Rare Diseases Therapeutics
Peptides can be strategically designed to target specific molecular pathways or genetic mutations associated with rare diseases. This precision-oriented approach allows for more effective and tailored therapeutic interventions. Peptides can be customized to address specific rare mutations or genetic variants, enabling treatments to be tailored to individual patients through personalized medicine approaches. Peptide-based therapies often offer non-invasive delivery options, making them an appealing choice for patients with rare diseases who may have limited tolerance for invasive procedures. Regulatory agencies provide incentives and special designations for drugs developed to treat rare diseases, creating opportunities for companies to pursue research and development in this field.Peptides offer promising solutions for the treatment of rare genetic disorders and orphan diseases. The expansion of the peptide synthesis market presents an opportunity to develop and manufacture peptide-based therapeutics targeting these conditions. Companies can focus on designing and synthesizing peptides that specifically address molecular targets associated with rare diseases, thus catering to unmet medical needs.
Key Market Players
- PolyPeptide Group
- Merck KGaA
- Thermo Fisher Scientific
- Enamine Ltd.
- Alfa Chemistry
- CEM Corporation
- GenScript
- AAPPTec
- Bachem Holding
- AnaSpec, Inc.
Report Scope:
In this report, the Global Peptide Synthesis Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Peptide Synthesis Market, By Product:
- Equipment
- Reagents and Consumables
- Others
Peptide Synthesis Market, By Technology:
- Solid-Phase Peptide Synthesis (SPPS)
- Solution-Phase Synthesis (SPS)
- Liquid-Phase Peptide Synthesis (LPPS)
Peptide Synthesis Market, By Application:
- Therapeutics
- Diagnosis
- Research
Peptide Synthesis Market, By End User:
- Pharmaceutical and Biotechnology Companies
- Contract Manufacturing Organization (CMO)
- Academic and Research Institutes
Peptide Synthesis Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Peptide Synthesis Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
Table of Contents
Companies Mentioned
- PolyPeptide Group
- Merck KGaA
- Thermo Fisher Scientific
- Enamine Ltd.
- Alfa Chemistry
- CEM Corporation
- GenScript
- AAPPTec
- Bachem Holding
- AnaSpec, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | August 2025 |
| Forecast Period | 2024 - 2030 |
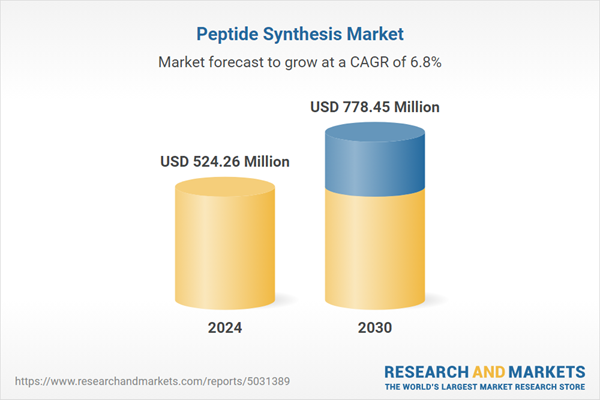
| Estimated Market Value ( USD | $ 524.26 Million |
| Forecasted Market Value ( USD | $ 778.45 Million |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |