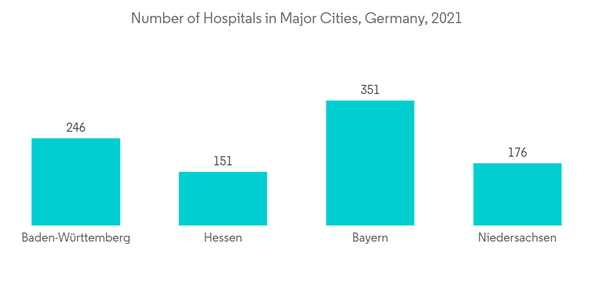
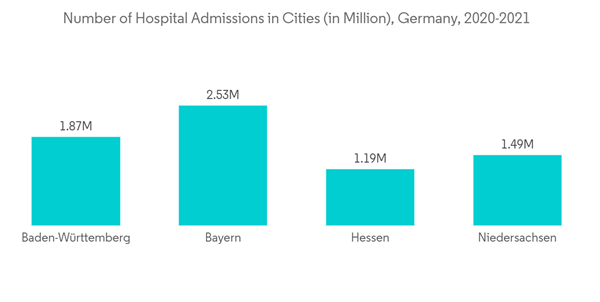
Key Highlights
- COVID-19 pandemic increased the demand for in-vitro diagnostic products in Germany due to the sharp rise in demand for polymerase chain reaction (PCR), next-generation sequencing (NGS), serology-based rapid-test products, the supportive regulatory landscape for product development and commercialization, and a rapid rise in target patient population which has boosted the market growth. Furthermore, rising initiatives from the key market players to distribute COVID-19 IVD tests propelled the market growth during the pandemic. For instance, in March 2020, Bruker Corporation entered a distribution agreement with Primer Design Ltd to distribute the CE-IVD-labeled genesig real-time PCR coronavirus (COVID-2019) assay in Germany. Thus, the rising tests and novel product launches boosted the market's growth during the pandemic. Therefore, the market slightly declined during the early pandemic due to supply chain restrictions and stringent lockdowns. However, the market gained a significant pace post-pandemic and is expected to show significant growth during the forecast period.
- The rising prevalence of chronic diseases, the increasing aging population, the surge in demand for accurate diagnosis, and favorable government initiatives are the key driving factors for the growth of the in-vitro diagnostics market in Germany. For instance, according to the study published in Experimental and Therapeutic Medicine in August 2021, the estimated incidence of ovarian cancer in Germany was reported to have a rate of 10.2 per 100,000. In addition, as per the 2021 report of the International Diabetes Federation (IDF), there were about 6.2 million people in Germany with diabetes. This number is projected to reach 6.5 million by 2030. Therefore, the rising incidence of chronic diseases such as cancers and diabetes is expected to drive the market study due to the increased adoption of in-vitro diagnostics for early detection.
- Furthermore, rising initiatives from the key market players are expected to propel the market growth over the forecast period. For instance, in May 2021, QIAGEN N.V. collaborated with Mirati Therapeutics Inc. to continue developing a tissue-based KRAS companion diagnostic to identify patients with cancers that have a KRASG12C mutation. The planned companion diagnostic would expand upon QIAGEN's therascreen KRAS testing portfolio based on real-time qualitative PCR for the QIAGEN Rotor-Gene Q MDx instrument, thereby increasing the adoption of QIAGEN Q MDx instrument, driving the market growth.
- Therefore, the rising incidence of chronic disease and the rising initiatives such as partnerships from the key market players are the factors expected to drive the market growth over the forecast period. However, stringent regulations coupled with cumbersome reimbursement procedures are a factor expected to hamper the market growth.
Germany In-Vitro Diagnostics (IVD) Market Trends
The Molecular Diagnostic Segment is Expected to Witness Healthy Growth Over the Forecast Period
- Molecular diagnostics are used to analyze biological markers in the genome and proteome to detect pathogens or mutations. Nucleic acid amplification technology for molecular diagnostics is the gold standard method for detecting viruses and has been increasingly adopted. The market players constantly focus on product launches based on nucleic acid amplification and product development strategies for COVID-19.
- This development is fueled by the introduction of powerful techniques, such as real-time polymerase chain reaction (PCR) and next-generation sequencing (NGS), which allow the amplification and decoding of genetic and epigenetic information.
- Furthermore, the rising awareness initiatives in molecular diagnostic screening are expected to drive the segment growth further. For instance, in May 2021, Seegene Germany GmbH took part in the government's Back-to-School program called the Lolli-Test, a starting point in returning our daily lives to normalcy. Under the program, Seegene Germany GmbH has supplied COVID-19 diagnostic tests to laboratories in business with the federal government, worth up to 19.3 million Euros. Such initiatives have led to increased adoption of molecular diagnostics tests in Germany, driving the market growth.
- Initiatives in strategies such as new product development, collaborations, acquisitions, and funding in research and development are adopted by key players to secure their positions in the German market. For instance, in March 2021, German company ZytoVision GmbH entered into a distribution agreement with Bio SB for the distribution of line ZytoVision of CE-approved chromogenic in situ hybridization and fluorescent in situ hybridization probes targeted for use in lung carcinomas.
- Hence, factors such as rising initiatives from the key market players, such as distribution of products and rising awareness of in-vitro diagnostic testing, are expected to drive the segment growth over the forecast period
Cancer/Oncology Among Applications is Expected to Hold a Significant Share in the Market Studied
- The factors driving the growth of the segment include the high burden on cancer, rising initiatives, and a strong foothold of key market players.
- According to the Statistisches Bundesamt (Destatis), in 2023, cancer is the fourth most frequent reason for treatment in Germany, accounting for 8% of all hospital stays with lung and bronchial cancer, particularly in common. Furthermore, according to the same source, in 2021, around 1.44 million patients were treated for cancer in hospitals on an in-patient basis in Germany. Such a huge burden of cancer in Germany is anticipated to drive market growth due to the increased adoption of IVDs for cancers.
- In addition, strategies such as new product development, collaborations, acquisitions, and funding in research and development are adopted by key players to secure their positions in the German market, driving segment growth. For instance, in March 2021, German company ZytoVision GmbH entered into a distribution agreement with Bio SB to distribute line ZytoVision of CE-approved chromogenic in situ hybridization and fluorescent in situ hybridization probes targeted for use in lung carcinomas.
- Hence, owing to the rising burden of cancer and key initiatives such as the distribution of products by market players is expected to drive the segment growth over the forecast period.
Germany In-Vitro Diagnostics (IVD) Industry Overview
The Germany in-vitro diagnostics market is fragmented with several market players. The market has rapidly evolved over the last few years. Industry observed significant changes in adopting market strategies such as product developments, mergers, and acquisitions in recent years. Thus, the German In-Vitro Diagnostics market has become a very competitive industry. Some of the players in the market include Abbott Laboratories, Becton, Dickinson and Company, Bio-Rad Laboratories Inc., bioMerieux SA, Danaher Corporation, Epigenomics Inc., F. Hoffmann-La Roche Ltd, Johnson & Johnson, Qiagen N.V., and Siemens Healthcare GmbH.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Becton, Dickinson and Company
- Bio-Rad Laboratories Inc.
- bioMerieux SA
- Danaher Corporation
- Epigenomics Inc.
- F. Hoffmann-La Roche Ltd
- Johnson & Johnson
- Qiagen N.V.
- Siemens Healthcare GmbH
- Seegene Germany GmbH