Global D-dimer Testing Market - Key Trends & Drivers Summarized
Why Is D-dimer Testing Crucial for Modern Healthcare Diagnostics?
D-dimer testing is an essential diagnostic tool used to detect blood clotting disorders, such as deep vein thrombosis (DVT) and pulmonary embolism (PE). This test measures the presence of D-dimer, a protein fragment that forms when a blood clot dissolves, indicating abnormal clotting activity within the body. It plays a critical role in the emergency and clinical settings, as it helps rule out serious conditions quickly and effectively. Given the rising incidence of cardiovascular diseases and venous thromboembolism (VTE), D-dimer testing has become increasingly vital in healthcare, ensuring timely intervention and improved patient outcomes. As the demand for efficient and accurate diagnostic solutions grows, the use of D-dimer tests is expanding across hospitals, diagnostic centers, and laboratories worldwide.How Are Technological Innovations Enhancing D-dimer Testing?
Technological advancements are transforming the D-dimer testing market, improving the accuracy, speed, and convenience of diagnostic processes. Innovations in point-of-care (POC) testing devices now allow for rapid D-dimer analysis in emergency settings, providing results in minutes and enabling quicker clinical decisions. Automated and high-sensitivity assay systems have also been developed, enhancing the precision of D-dimer measurements and minimizing false positives. Integration of D-dimer testing into multiplex testing platforms that combine multiple biomarkers is further enhancing diagnostic capabilities, particularly for detecting and managing complex conditions like COVID-19-related coagulopathies. These innovations are not only increasing the effectiveness of D-dimer testing but are also expanding its adoption across various healthcare settings, contributing to market growth.What Trends Are Driving the Adoption of D-dimer Testing in Healthcare Facilities?
The increased focus on early diagnosis and preventive care is driving the adoption of D-dimer testing in healthcare facilities. With cardiovascular diseases remaining a leading cause of mortality globally, healthcare providers are increasingly utilizing D-dimer tests as a quick and reliable method to screen for thrombotic disorders. The demand for POC diagnostic solutions is also boosting market growth, as these tests enable healthcare professionals to conduct assessments in emergency rooms, intensive care units, and remote locations. Furthermore, the ongoing impact of the COVID-19 pandemic has highlighted the importance of coagulation testing, leading to a surge in demand for D-dimer tests to monitor and manage coagulopathies associated with the virus. This trend is expected to continue as healthcare systems prioritize enhanced diagnostic capabilities.What Factors Are Driving Growth in the D-dimer Testing Market?
The growth in the D-dimer testing market is driven by several factors, including the rising prevalence of cardiovascular and thrombotic diseases, advancements in POC testing technology, and the expanding need for quick diagnostics in emergency care settings. The global increase in chronic conditions such as diabetes and obesity, which are associated with higher risks of blood clots, is further boosting the demand for D-dimer tests. Technological improvements, such as automated and high-sensitivity assays, are enhancing test accuracy and efficiency, encouraging healthcare facilities to integrate these systems into their diagnostic protocols. Additionally, the impact of the COVID-19 pandemic has elevated the importance of coagulation testing, driving investments in D-dimer diagnostic solutions. The combination of these factors is fueling the expansion of the D-dimer testing market across hospitals, laboratories, and POC settings.Report Scope
The report analyzes the D-dimer Testing market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Reagents & Consumables, Analyzers); Test Type (Clinical Laboratory Tests, Point-of-Care Tests); Application (Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), Disseminated Intravascular Coagulation (DIC), Other Applications); End-Use (Hospitals, Diagnostic Centers, Academic & Research Institutes, Other End-Uses).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Reagents & Consumables segment, which is expected to reach US$1.3 Billion by 2030 with a CAGR of a 3.6%. The Analyzers segment is also set to grow at 4.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $425.9 Million in 2024, and China, forecasted to grow at an impressive 3.8% CAGR to reach $317.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global D-dimer Testing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global D-dimer Testing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global D-dimer Testing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Inc., Alere, Inc., Beckman Coulter, Inc., Becton, Dickinson and Company, Bio/Data Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 11 companies featured in this D-dimer Testing market report include:
- Abbott Laboratories, Inc.
- Alere, Inc.
- Beckman Coulter, Inc.
- Becton, Dickinson and Company
- Bio/Data Corporation
- Corgenix
- F. Hoffmann-La Roche AG
- Helena Biosciences Europe
- Siemens Healthcare GmbH
- Sysmex Corporation
- Thermo Fisher Scientific, Inc.
- Trinity Biotech PLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories, Inc.
- Alere, Inc.
- Beckman Coulter, Inc.
- Becton, Dickinson and Company
- Bio/Data Corporation
- Corgenix
- F. Hoffmann-La Roche AG
- Helena Biosciences Europe
- Siemens Healthcare GmbH
- Sysmex Corporation
- Thermo Fisher Scientific, Inc.
- Trinity Biotech PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 158 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
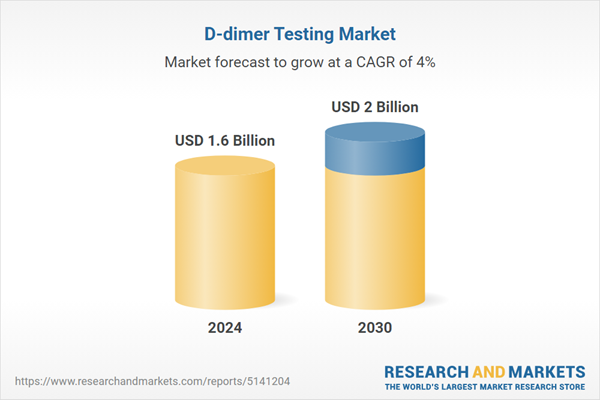
| Estimated Market Value ( USD | $ 1.6 Billion |
| Forecasted Market Value ( USD | $ 2 Billion |
| Compound Annual Growth Rate | 4.0% |
| Regions Covered | Global |