The COVID-19 pandemic significantly impacted South Korea's in-vitro diagnostic market. For instance, an article published by ERMPS in January 2022 reported the high prevalence of infectious diseases in South Korea during the COVID-19 pandemic. Thus, initially, the high prevalence of infectious diseases and increasing COVID-19 cases in South Korea surged the demand for IVD in the country. In the current scenario, it is anticipated with a substantial decrease in COVID-19 cases due to the increase in COVID-19 vaccination; the demand for IVD kits may get reduced as compared to the initial pandemic times. However, due to the growing burden of other infectious and chronic diseases, the studied market is expected to witness stable growth over the next 3-4 years.
The factors that are driving the growth of the studied market are:
Key Highlights
- The growing burden of chronic disease.
- Increasing use of point-of-care diagnostics.
- Raising awareness and acceptance of personalized medicine and companion diagnostics.
For instance, an article published in the journal Cancer Res Treat. in April 2022 reported that 274,488 new cancer cases are anticipated to occur in 2022. The most common cancer site is expected to be the thyroid, followed by the lung, colon and rectum, breast, and stomach. These five cancers are expected to represent half of Korea's overall cancer burden. Thus, the high prevalence of chronic diseases like cancer is increasing the demand for IVD testing, thereby driving the growth of the studied market.
Moreover, with the technological advancement in the various in-vitro diagnostics technologies, the use of these tests is increasing rapidly due to better results in less time and cost, thus driving South Korea's IVD market. For instance, in January 2022, South Korea's molecular diagnostic company, Seegene Inc., launched the Allplex SARS-CoV-2 fast PCR assay, which can deliver PCR results in just 60 minutes. Thus, such product launches are propelling the growth of the studied market.
Furthermore, the increasing awareness and acceptance of personalized medicine and companion diagnostics are fueling the studied market's growth. For instance, in June 2022, Agilent Technologies Inc reported South Korea's MFDS had approved the company's PD-L1 IHC 22C3 pharmDx as a companion diagnostic (CDx). It will identify patients with non-small cell lung cancer suitable for first-line monotherapy with KEYTRUDA (pembrolizumab) on the Dako Omnis platform. Thus, such approvals and product launches are fueling South Korea's IVD market.
So, due to the growing burden of chronic diseases, increasing use of point-of-care diagnostics, and increasing awareness and acceptance of personalized medicine and companion diagnostics, the studied market is expected to witness significant growth over the forecast period. However, stringent regulations may slow down the development of the studied market.
South Korea In Vitro Diagnostics Market Trends
Reagents By Product are Expected to Witness Significant Growth Over the Forecast Period.
The reagent segment is anticipated to witness lucrative growth over the forecast period owing to the increasing use of chemical, biological, or immunological components, solutions, or preparations intended by the manufacturers to use during the in-vitro diagnosis process.Furthermore, the COVID-19 outbreak has fueled the demand for in-vitro diagnostic reagents, as several market players in the country are engaged in implementing strategic initiatives, thereby contributing to market growth. For instance, in September 2021, a South Korean company, Seegene, launched the Novaplex SARS-CoV-2 Variants V Assay. It is a new concept diagnostic reagent that detects the Delta and Lambda variants. Such developments will likely supplement the market's growth throughout the analysis period.
Similarly, in June 2022, Parse Biosciences partnered with Molecular Diagnostics Korea (MDxK) to expand its presence in Asia by providing its entire line of Evercode Whole Transcriptome products and Cell and Nuclei Fixation kits in South Korea. MDxK provides instruments, consumables, and other services to support molecular research.
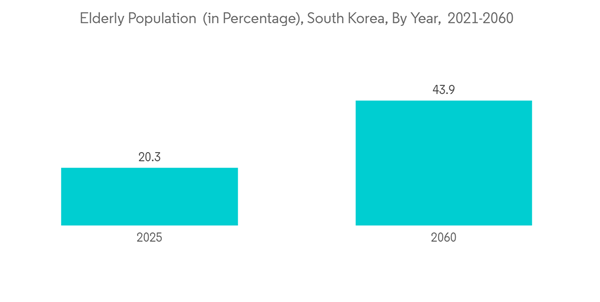
Also, as per the data published by the National Statistical Office Korea, in 2021, 16.5% of the total population was 65 years of age or older due to the rapidly aging society. The percentage is anticipated to rise, even more, reaching 20.3% in 2025 and 43.9% in 2060. With the increasing burden of the geriatric population in the country, the burden of chronic diseases in South Korea is also expected to rise. It, in turn, is anticipated to drive the demand for IVD tests used in diagnosing chronic diseases. As a result, the use of reagents across various IVD testing is also anticipated to increase over the coming years.
Thus, due to the increasing use of chemical, biological, or immunological components and solutions, the growing burden of chronic diseases, the rising geriatric population, and technological advancements, the segment is anticipated to witness significant growth over the forecast period.
Cancer/Oncology Segment is Expected to Witness Significant growth Over the Forecast Period.
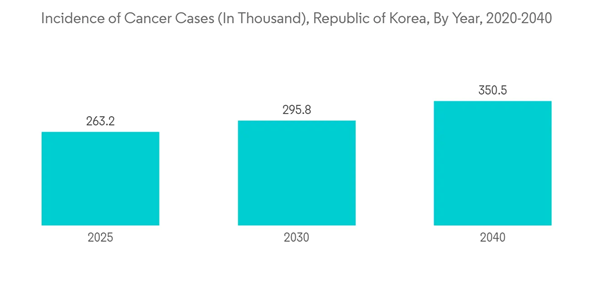
The cancer segment is expected to witness significant growth over the forecast period due to the increasing number of cancer cases reported in South Korea. For instance, in 2020, the International Agency for Research on Cancer reported 230,000 cases were recorded in the Republic of Korea 2020. This number is expected to increase to 350,000 by 2040. Thus, the increasing number of cancer cases is expected to increase the demand for IVD instruments for cancer diagnosis, thereby driving the growth of the studied market.Similarly, an article published by the journal JGO in February 2022 reported that the cervical cancer screening rate was 56.0% in 2021 among South Korean women. The article said the high prevalence of cervical cancer in South Korean women and the rising awareness among them is constantly propelling the demand for IVD kits, thereby contributing to market growth.
Furthermore, South Korea's government initiative to control cancer cases by assisting with cancer testing is also contributing to the growth of this segment. For instance, in October 2022, National Cancer Center Korea reported it initiated the Vision 2020 program. It represents specific guidelines to protect the Korean people from cancer through innovative research, quality medical care, optimal cancer expert training, and national cancer control programs support. Thus, such initiatives are leading to increasing cancer diagnoses, thereby driving the growth of the studied market.
Thus, the rising cancer cases are leading to increasing demand for IVDs and driving the growth of South Korea's in-vitro diagnostics market.
South Korea In Vitro Diagnostics Market Competitor Analysis
South Korea's in-vitro diagnostics market is moderately competitive and consists of several significant players operating globally and regionally. The competitive landscape includes an analysis of a few international and local companies holding market shares and are well known, such as Abbott Laboratories, Becton, Dickinson and Company, Bio-Rad Laboratories Inc., Danaher Corporation, F. Hoffmann-La Roche AG, Siemens Healthineers, Sysmex Corporation, Thermo Fisher Scientific Inc., Seegene Inc., and Gencurix.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Becton, Dickinson and Company
- Bio-Rad Laboratories Inc.
- Danaher Corporation
- F. Hoffmann-La Roche AG
- Siemens Healthineers
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- Seegene Inc.
- Gencurix