The COVID-19 pandemic significantly affected the invitro diagnostic market in the Netherlands owing to factors such as delayed laboratory appointments and reduced diagnostics. For instance, as per an article published in February 2022 by PubMed, the largest decrease in diagnostics was seen during the first lockdown, when the number of pathology reports declined up to 88%, and almost all specimen types were affected in the Netherlands. Pathologists were among them, receiving fewer specimens than usual, particularly during the lockdown. However, with restrictions lifted, the invitro diagnostics market is likely to witness growth in the coming years due to the rise in chronic disease, increase in testing laboratories, and demand for point-of-care testing.
The major factors fuelling this market include a rapid increase in chronic diseases, increasing demand for point-of-care testing and personalized medicine, and technological advances, along with the rising geriatric population in the country. For instance, according to VZinfo, in 2021, 53,100 new patients with diabetes were estimated to be diagnosed in the Netherlands. This estimated 29,600 men and 23,500 women (3.4 per 1,000 men and 2.7 per 1,000 women). In almost all age groups, the number is higher for men than for women. In addition, patients can deal with their conditions better with the help of IVDs (diabetic patients can use portable blood glucose monitors regularly to determine their blood glucose level). Furthermore, as per a Centraal Bureau Voor de Statistiek report in 2021, half of Dutch adults were overweight and 13.9% were severely overweight or obese and more prone to chronic diseases. The demand for IVD products is influenced by the increase in incidences of chronic diseases, thereby pushing market growth. Furthermore, government schemes to reduce the cost of IVD procedures in the Netherlands are also anticipated to promote market growth. For instance, according to the European Diagnostic Manufacturers Association (EDMA), the cost of IVDs is less than EUR 21 per person per year.
Netherlands In Vitro Diagnostics Market Trends
The Molecular Diagnostics Segment is Expected to Register Significant Growth in the Netherlands In Vitro Diagnostics Market Over the Forecast Period
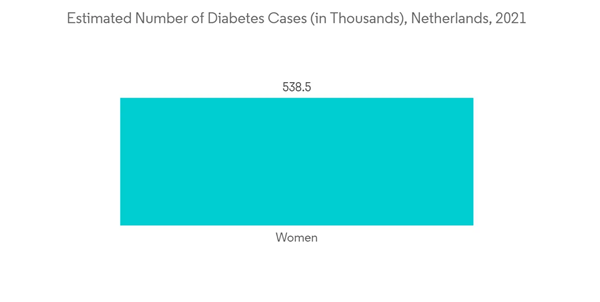
The molecular diagnostic segment is anticipated to witness growth in the market owing to the factors such as a rise in chronic diseases that require early diagnosis and the increasing demand for point-of-care testing and personalized medicine. Molecular diagnostic devices are used to analyze biological markers in the genome and proteome to detect pathogens or mutations. Additionally, the incidence of cancer in the Netherlands is also expected to drive the growth of molecular diagnostics owing to the growing demand for effective diagnostics. For instance, as per the Netherlands Comprehensive Cancer Organisation (IKNL), in 2032, there are likely to be about 156,000 new diagnoses of cancer. This means that in less than ten years, an average of 18 people per hour will be diagnosed with cancer.Furthermore, according to VZinfo, in 2021, an estimated 1,156,900 people with diabetes were living in the Netherlands (The number of people who have had a particular disease during a given year). These were 618,300 men and 538,500 women (70.9 per 1,000 men and 61.1 per 1,000 women). Diabetes is more common in men than women in almost all age groups in the Netherlands. Thus, in some cases due to the genetic etiology of monogenic diabetes, molecular diagnostics can be used for diagnosis and classification.
New product launches in the Netherlands are also expected to drive the growth of the studied segment during the forecast period. For instance, in August 2022 IncellDx received the CE marking for a long-COVID blood test that will be launched in the European markets. Similarly, in December 2021 PathoFinderand EWC Diagnostics partnered to rapidly advance the expansion of COVID-19 testing capacity in the Netherlands by providing the Dutch government with complete sets of sampling materials and SARS-CoV-2 PCR kits.
The Infectious Disease Segment is Expected to Register the Fastest Growth in the Netherlands IVD Market Over the Forecast Period
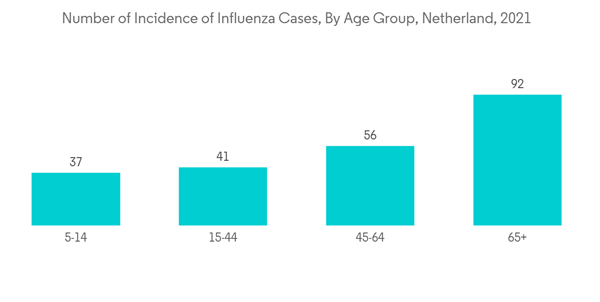
The infectious disease segment is anticipated to witness the fastest growth in the market owing to the factors such as a rise in infectious diseases across the country. For instance, per a Communicable Disease Threats Report in November 2022, the number of monkeypox cases reported in the Netherlands was 1,248 in November 2022, whereas the count was 1,221 in September 2022. As per the same source, 4 cases of diphtheria were reported in 2022. Furthermore, per a 2022 update from VZinfo.nl, the incidence of influenza cases in the Netherlands in 2021 was 341 for the age group 0 to 4 years.Furthermore, laboratory services launches and government initiatives to eradicate infectious diseases are likely to boost the market growth. For instance, in May 2022, Eurofins Scientific, a company focused on bioanalytical testing, signed an agreement with Stichting PAMM Laboratoria voor Pathologie en Medische Microbiologie, a medical microbiology and pathology laboratory diagnostics company in the Netherlands. PAMM serves six hospitals in the Netherlands, including two top-rated clinical oncology centers, general practitioners, and independent treatment centers. The lab specializes in medical microbiology and pathology and was the first in the Netherlands to have implemented full digital pathology.
Netherlands In Vitro Diagnostics Industry Overview
The Netherlands in vitro diagnostics market is moderately competitive, with several key players, such as Roche Diagnostics, Abbott Laboratories, Bio-Rad Laboratories Inc., Danaher Corporation, and Thermo Fisher Scientific Inc., holding a large share of this market.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Becton, Dickinson and Company
- Bio-Rad Laboratories Inc.
- Danaher Corporation
- Johnson & Johnson
- Roche Diagnostics
- Siemens Healthcare
- Thermo Fisher Scientific Inc.