The COVID-19 pandemic impacted the market significantly. Due to restricted hospital visits and delayed surgeries, the demand for MIS devices declined and hampered the market's growth during the initial phase of the pandemic. However, the lockdowns and restrictions were lifted, and the surgical procedures started operating following specialized regulations from the beginning of 2021. For instance, according to an article published in July 2022 in the Global Journal of Medical Pharmaceutical and Biomedical Update, minimal access approaches were found safe during the COVID-19 pandemic with proper patient selection and additional precautions. As MIS reduces the hospital stay, it needs to be considered as a means of decreasing disease transmission. Hence, developing and implementing proper guidelines has undeniably minimized the incidence of COVID-19 infection during the later phases. Consequently, the pandemic considerably affected the demand generation of MIS, which may continue in the future. Thus, as per the analysis, the market's growth is expected to increase and reach pre-pandemic levels during the forecast period.
Factors such as an increasing prevalence of lifestyle-related and chronic disorders and a rising preference for minimally invasive procedures are the major contributors to the market's growth.
MIS has several advantages, such as fewer incisions, less chance of infection, and rapid recovery time. Therefore, physicians recommend procedures to treat chronic disorders such as gynecological, urological, gastrointestinal, and others due to such advantages. Thus, the adoption of these surgeries is increasing. For instance, according to the article published in the Annals of Medicine and Surgery Journal in October 2021, multiple facets of the conventional surgical method have been replaced by MIS while gaining interest due to improved survival, fewer complications, and rapid recoveries in recent years. Therefore, an increasing number of MIS is anticipated to propel the market's growth in the future.
Global and regional market players are developing and innovating advanced products in the country. For instance, in April 2021, Ambu Inc. received approval from Health Canada for aScope 4 Cysto, a single-use endoscopes platform for urology. In addition, in April 2021, Olympus Corporation, in collaboration with Veran Medical Technologies Inc., launched the US bronchoscopy portfolio of the 510(k)-cleared H-SteriScope Single-Use Bronchoscopes, a line of five premium endoscopes for use in advanced diagnostic and therapeutic procedures. Thus, such activities are anticipated to create opportunities for MIS device innovation and development and fuel the market's growth over the forecast period.
However, the lack of skilled professionals and the high cost of minimally invasive surgeries and devices are expected to hinder the market's growth over the forecast period.
Minimally Invasive Surgery Devices Market Trends
Endoscopic Devices is Expected to be the Fastest-growing Segment During the Forecast Period
Endoscopic devices are minimally invasive and can be inserted into natural openings of the human body to observe an internal organ or a tissue in detail. The surgeries performed using endoscopic devices are generally imaging procedures and minor surgeries. Endoscopy can be indicated for various types of conditions, such as gastrointestinal disorders, pancreatitis, gastric/stomach cancer, respiratory tract disorders, urinary tract disorders, etc. Due to the clinical significance of endoscopic devices, these devices are gaining market demand and are expected to continue their growth at a faster rate over the next few years.Endoscopic devices pose various advantages for the detection of various diseases. For instance, as per an article published in December 2021 in Cancers Journal, the detection and diagnosis of stomach lesions have improved due to the ongoing advancements in endoscopic technologies over time. Endoscopic technologies have been continuously advancing to facilitate improvement in the detection and diagnosis of gastric lesions. Thus, owing to the burden of cancer globally, the demand for endoscopic devices is likely to rise. This is expected to propel the endoscopic devices segment during the forecast period.
The market for an endoscope is expected to be driven by favorable reimbursement policies, technological advancements, and an increasing number of new players in the endoscopy devices market. As per the September 2022 update by KARL STORZ, the company manufactures best-in-class endoscopes in Sydney, Australia. In addition to the Sydney location, the company operates in more than 40 countries worldwide. Thus, the presence of such global and regional manufacturers focused on developing innovative endoscopes is anticipated to garner the segment's growth during the forecast period.
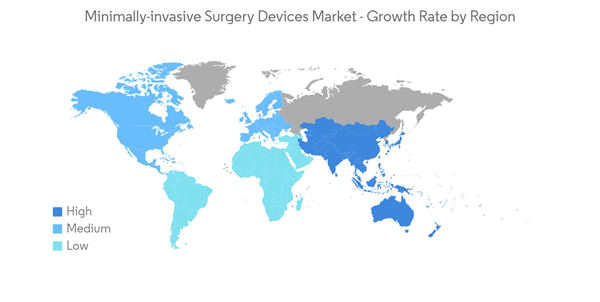
North America is Expected to Hold a Significant Share of the Market Over the Forecast Period
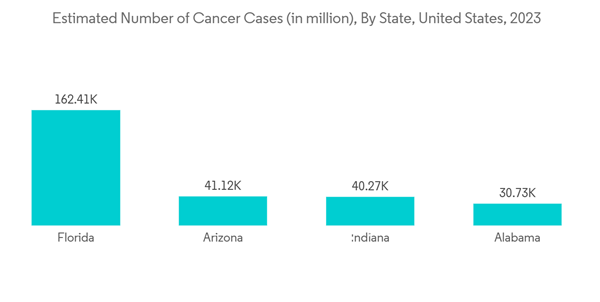
North America held a major share of the minimally invasive surgery devices market in the past few years, and it is expected to show a similar trend over the forecast period, primarily due to the high burden of diseases requiring minimally invasive procedures, increasing awareness about MIS.In North America, the United States is expected to hold a major market share during the forecast period due to the high prevalence of chronic diseases such as cardiovascular diseases, cancer, and neurological diseases. For instance, according to the data updated by CDC in October 2022, every 34 seconds, a person suffers from cardiovascular disease in the United States, showing a high burden of the disease in the country, creating the need for surgeries. This is expected to create opportunities for minimally invasive surgical procedures in the country and drive the market's growth over the forecast period.
Technological advancements in various surgical devices used for MIS developed by key market players and new device launches are further expected to complement the growth of the market in the region. For instance, in May 2021, the Food and Drug Administration approved the NaviCamTM Magnetically Controlled Capsule Endoscopy (MCCE) system for premarketing. In addition, in March 2021, Misonix Inc., a provider of minimally invasive therapeutic ultrasonic medical devices and regenerative products, received a Health Canada license for the neXus Ultrasonic Surgical System. neXus is a powerful, highly-integrated, and easy-to-use system incorporating the latest advances in ultrasonic technology for increased efficiency and efficacy in treating procedures across various surgeries, including laparoscopic surgery. Thus, such product launches are anticipated to fuel the market's growth in the region over the forecast period.
Minimally Invasive Surgery Devices Industry Overview
The minimally invasive surgery devices market is competitive and fragmented, owing to the presence of various key players in the market. Many market players are investing significant resources in new product innovation, offering novel devices to treat patients, and delivering high-quality and life-sustaining treatment. Some of the market players are Abbott Laboratories, HOYA Corporation, Intuitive Surgical Inc., Koninklijke Philips NV, Medtronic PLC, Olympus Corporation, Boston Scientific, and Stryker.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Boston Scientific
- Intuitive Surgical Inc.
- Koninklijke Philips NV
- Medtronic PLC
- Olympus Corporation
- HOYA Corporation (Microline Surgical)
- Steris
- Stryker Corporation
- Zimmer Biomet Holdings Inc.
- Johnson & Johnson Inc.
- Renishaw PLC
- CONMED Corporation