The healthcare system witnessed enormous challenges as a result of the COVID-19 pandemic. All outpatient treatments were postponed or restricted during the COVID-19 pandemic to reduce the risk of viral transmission, as most chronic therapies were regarded as non-urgent. For instance, as per the article published in November 2022 in PubMed, during the early stages of the COVID-19 pandemic, catheter ablation (CA) significantly decreased before gradually increasing to pre-COVID-19 levels. Due to the suspension of all non-urgent elective surgical operations during the COVID-19 pandemic, the number of ablation surgeries performed during the period is very low compared to that of the previous year. However, the market is anticipated to witness growth in the coming years due to the rise in the prevalence of chronic diseases and an increase in product sales.
Major chronic diseases such as cancer, cardiovascular diseases, and other ophthalmologic, gynecological, and urological diseases are on the rise all over the world, which is contributing to the growth of the ablation devices market. With increasing risks, there are also effective surgical therapies that lead to the efficient treatment of patients suffering from chronic illnesses. Furthermore, the increasing number of factors contributing to the rise in chronic disease is increasing the market share for ablation devices.For instance, as per the American Cancer Society, the number of people estimated to be diagnosed with cancer is likely to be 1.9 million in 2022 in the United States. Also, according to the International Diabetes Federation's Diabetes Atlas, Tenth Edition, for 2021, in 2021, around 537 million adults all over the world were found to have diabetes, with the numbers projected to grow to 643 million by 2030 and 783 million by 2045. In individuals with diabetes mellitus (DM), catheter ablation of atrial fibrillation (AF) appears to be a safe therapy that considerably lowers symptom load. Additionally, as per the Elective Surgery Activity Data published by AIHW, the number of patients added for elective surgery in 2020-2021 (893,200) was a 6.6% increase in the number of additions from the previous year. Hence, with the rise in the number of chronic diseases coupled with an increase in elective surgery cases, the market studied is expected to grow over the forecast period.
Several research and development studies focusing on innovative ablation devices are also contributing to the growth of the blood flow device measurement market. For instance, in May 2021, Acutus Medical initiated a prospective, multi-center, non-randomized global clinical study designed to demonstrate the safety and performance of the AcQBlate Force Sensing System. The AcQBlate Force Sensing System is a complete and differentiated solution for the electrophysiologic mapping and radiofrequency ablation of cardiac arrhythmias. The study focuses on the catheter ablation of accessible pulmonary veins with the endpoint of creating electrical isolation for each targeted vein and is expected to be completed by January 2024. The increasing research in the development of ablation devices is expected to boost the growth of the ablation devices market in the forecast period.
The increasing developments made among the key players in the ablation segment are also contributing to the growth of the market. For instance, in November 2021, Hologic, Inc., launched the NovaSure V5 global endometrial ablation (GEA) device, the most studied and trusted endometrial ablation procedure in the United States. The rise in the research and development of such devices is expected to boost market growth.
Thus, due to the rise in chronic diseases, increase in product launches, and research associated with ablation devices, the studied market is anticipated to witness growth over the forecast period. However, the high cost of the devices and the strict regulations, coupled with the risk associated with the ablation procedures, are likely to hinder the market's growth.
Ablation Devices Market Trends
The Cancer Treatment Segment is Expected to Hold a Significant Market Share in the Ablation Devices Market Over the Forecast Period
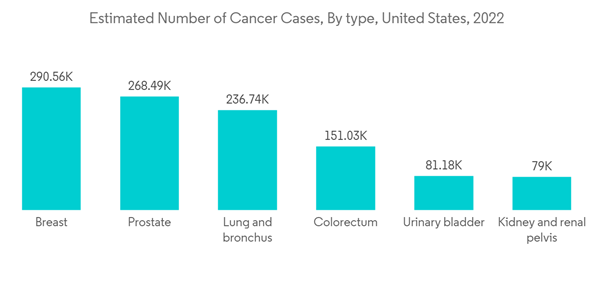
Radiofrequency ablation is one of the most common ablation methods for small tumors. It uses high-energy radio waves. Special probes are used to 'burn' or 'freeze' cancers without the usual surgery. Computed tomography (CT), ultrasound (US), or magnetic resonance imaging (MRI) is used to guide and position the needle probe into the tumor. The cancer treatment segment is anticipated to hold a significant share of the ablation devices market owing to the rising number of cancer cases around the world. For example, according to IARC estimates, there will be 28.9 million cancer cases worldwide by 2040. The large increase in the number of cases over the years is majorly contributing to the increase in cancer surgical therapies for efficient treatment studies, which in turn will propel the growth of the ablation devices market in the forecast period.According to an article by RadiologyInfo.org updated in February 2021, radiofrequency ablation (RFA), a minimally invasive treatment for cancer, employs an image-guided technique to apply heat to destroy cancer cells. RFA uses ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) to guide a needle electrode into a cancerous tumor and passes high-frequency electrical currents through the electrode to ground pads placed on the body, thereby creating focused heat that destroys the cancer cells surrounding the electrode. The article also said that radio frequency and microwave ablations are the most common ablation treatments for cancer therapy.
Several market players are also focusing on ablation devices for cancer treatment. Avenda Health, for example, received FDA 510(k) clearance for its cancer management platform, iQuest, in December 2022.In order to produce a customized map of the prostate cancer's location, iQuest integrates already-available patient-specific diagnostic data with deep-learning algorithms. This will, for the first time, enable more individual, efficient, and accurate therapies. Instead of treating the entire organ, the platform enables a doctor to make choices that take cancer's extent into account. The selection, planning, guidance, and follow-up of treatments are made easier for doctors and patients with the use of this information. iQuest can be applied to multiple treatment options to guide a doctor's intervention plan, including FocalPoint, Avenda Health's soft tissue laser ablation device, and other focal treatments; active surveillance; and decision-making for whole gland treatments, including radical prostatectomy and radiation therapy. Such research and developments in ablation devices are expected to propel market growth over the forecast period.
Thus, the studied segment is expected to grow in the market over the next few years because there are more chronic diseases, more new products are coming out, and more research is being done on ablation devices.
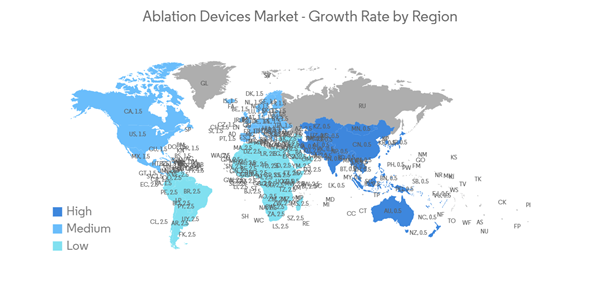
North America is Expected to Hold a Significant Share in the Market and Expected to do Same Over the Forecast Period
The rise in the adoption of highly advanced techniques and systems in the manufacturing of ablation devices and the technological advancements made in ablation device studies are expected to boost the growth of the market in the North American region. People in the area know a lot about the new ablation device therapies that are on the market, which helps the market grow quickly in the area.The rising number of chronic disease cases in the North American region is contributing to the growth of the ablation devices market. For instance, as per the Statistics Canada report, an estimated 233,900 people in Canada are likely to be diagnosed with cancer in 2022. Furthermore, as per the Government of Canada update, lung, breast, colorectal, and prostate cancers were expected to remain the most commonly diagnosed cancers, accounting for 46% of all diagnoses in 2021. The large population affected by chronic diseases and the increase in the necessity of ablation devices for the ease of diagnosis and treatment of such diseases are expected to propel the growth of the ablation devices market.
Also, the rise in the development of innovative ablation devices in the region is contributing to the growth of the market. For instance, in January 2022, Medtronic plc entered into a definitive agreement to acquire Affera, Inc. The acquisition expands the Medtronic portfolio of advanced cardiac ablation products and accessories to meet physician needs within a growing patient population. Similarly, in February 2022, Medtronic plc received FDA approval for its Freezor and Freezor Xtra Cardiac Cryoablation Focal Catheters, which are the first and only ablation catheters to treat the growing prevalence of pediatric Atrioventricular Nodal Reentrant Tachycardia (AVNRT). The key players' strategic acquisitions and new product launches are likely to make the product more available and boost the growth of the ablation devices market over the next few years.
Thus, the growth of the market in this region over the next few years will be driven by the growing need for ablation devices and the key players' increasing number of strategic initiatives and new product launches.
Ablation Devices Market Competitor Analysis
The global Ablation Device Market is moderately fragmented and consists of a few major players. Companies like Abbott, AngioDynamics, Inc., AtriCure, Inc., Boston Scientific Corporation, BTG plc, Conmed Corporation, Johnson & Johnson, Medtronic PLC, Olympus Corporation, and Smith & Nephew PLC, among others, hold a substantial market share in the ablation devices market.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Alcon Laboratories
- AngioDynamics, Inc.
- AtriCure, Inc.
- Bausch & Lomb Incorporated
- Biotronik
- Boston Scientific Corporation
- Conmed Corporation
- Johnson and Johnson
- Medtronic PLC
- Olympus Corporation
- Smith & Nephew PLC