Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative introduction to diuretics that frames clinical relevance, evolving therapeutic differentiation, and the operational pressures shaping industry decisions
The diuretics therapeutic area remains a foundational pillar of medicines used across cardiology, nephrology, ophthalmology, and general medicine, delivering established mechanisms of action that address fluid balance, intraocular pressure, and blood pressure control. As patient populations age and the prevalence of comorbid conditions persists, clinical reliance on diuretics endures, but the landscape is evolving in response to new clinical guidelines, tightened regulatory scrutiny, and technological advances in drug delivery and patient monitoring.
Recent years have seen an increasing emphasis on therapeutic differentiation within a largely genericized drug class, prompting manufacturers to explore formulation improvements, fixed-dose combinations, and companion diagnostics to reinforce clinical value. Meanwhile, clinicians are navigating heightened expectations for safety, particularly in vulnerable populations such as those with renal impairment or multi-drug regimens, which has elevated the importance of real-world evidence and post-marketing surveillance.
Transitioning from historical usage patterns, the market now reflects a balance between cost containment pressures and opportunities to demonstrate superior patient outcomes through targeted research and commercial strategies. Consequently, industry participants must align clinical evidence with pragmatic distribution and manufacturing choices, while payors and providers seek demonstrable value that supports formulary placement and long-term adherence.
How clinical mandates, supply chain modernization, regulatory pressure, and digital health innovation are jointly reshaping the future of diuretics delivery and adoption
The diuretics landscape is undergoing transformative shifts driven by intersecting clinical, technological, and policy forces that are redefining how products are developed, delivered, and adopted. On the clinical front, updated treatment algorithms and growing awareness of polypharmacy risks have pushed prescribers to evaluate diuretic selection more holistically, integrating renal function monitoring and comorbidity management into prescribing decisions. This clinical tightening amplifies demand for robust evidence packages that clarify comparative safety and efficacy across patient subgroups.
Concurrently, supply chain modernization and manufacturing innovation are encouraging a movement away from single-source dependencies toward distributed production models and strategic inventory bufferization. Technological advances in formulation science and drug-delivery systems are enabling extended-release profiles and parenteral formulations tailored to acute care settings, while digital health solutions increasingly support adherence, adverse event reporting, and remote monitoring.
From a commercial standpoint, payor emphasis on outcomes has elevated the importance of health economics analyses and real-world data generation as negotiation tools. In addition, evolving global trade policies and procurement practices are incentivizing nearshoring and regional supplier development, prompting strategic reassessments of manufacturing footprints. Taken together, these shifts are fostering a more evidence-driven, resilient, and technology-enabled diuretics ecosystem.
Assessing the cumulative operational, procurement, and supply chain consequences of U.S. tariff changes in 2025 on diuretics sourcing, manufacturing, and distribution
The introduction of new tariff measures by the United States in 2025 has created a ripple effect across the diuretics supply chain, influencing sourcing strategies, manufacturing choices, and procurement practices without altering the fundamental clinical value of the therapies. Import duties on select pharmaceutical inputs elevated the cost calculus for firms that relied heavily on internationally sourced active pharmaceutical ingredients and finished dosage forms, prompting a reassessment of long-standing supplier relationships and logistics models.
In response, many organizations pursued diversification strategies, exploring secondary sourcing, expanded qualification of regional suppliers, and strategic inventory positioning to mitigate exposure. Regulatory and customs authorities in both exporting and importing jurisdictions also adjusted procedural requirements and tariff classifications, which in turn required legal and compliance teams to reassess classification strategies and documentation practices. These administrative changes introduced short-term complexity for manufacturers and distributors as they navigated updated import protocols and cost-to-serve calculations.
Moreover, procurement entities such as hospitals and large pharmacy chains reacted by intensifying supplier performance evaluations and by seeking contractual mechanisms to share or offset tariff-driven cost fluctuations. Simultaneously, the policy environment spurred renewed interest in domestic capacity building, with producers and investors evaluating the trade-offs between higher manufacturing costs and improved control over supply continuity. Ultimately, the cumulative impact has been a strategic refocusing on resilience and flexibility rather than a fundamental shift in therapeutic utilization.
Deep segmentation insights linking drug class, indication, administration route, dosage form, and distribution channels to inform targeted development and commercialization strategies
A nuanced segmentation view reveals where clinical demand, manufacturing complexity, and distribution priorities intersect, informing targeted commercial and development strategies. Based on drug class, the landscape encompasses Carbonic Anhydrase Inhibitors studied across Acetazolamide and Dorzolamide, Loop Diuretics studied across Bumetanide, Ethacrynic Acid, Furosemide, and Torasemide, Osmotic Diuretics studied across Mannitol, Potassium-Sparing Diuretics studied across Amiloride, Eplerenone, Spironolactone, and Triamterene, and Thiazide Diuretics studied across Bendroflumethiazide, Chlorthalidone, and Hydrochlorothiazide. Each class has distinct clinical use cases, safety considerations, and formulation preferences that influence development priorities and lifecycle decisions. Based on indication, therapeutic deployment spans Edema, Glaucoma, Heart Failure, Hypertension, and Renal Disease, and these clinical contexts dictate differential dosing regimens, monitoring requirements, and caregiver education needs. Based on route of administration, adoption patterns split between Oral and Parenteral forms, with oral formulations driving chronic outpatient management and parenteral options serving acute inpatient needs where rapid diuresis or intraocular pressure control is required. Based on dosage form, the market is served by Capsule, Injectable, Liquid, and Tablet presentations, each presenting distinct manufacturing and stability challenges as well as patient adherence considerations. Based on distribution channel, the products flow through Hospital Pharmacy, Online Pharmacy, and Retail Pharmacy networks, with each channel imposing different reimbursement models, stocking practices, and stakeholder expectations.
Taken together, these segmentation layers create intersecting decision nodes for manufacturers and commercial teams. For example, a loop diuretic intended for inpatient acute care will prioritize injectable stability and hospital distribution agreements, whereas a thiazide aimed at chronic hypertension management will prioritize oral tablet bioequivalence, affordability, and retail pharmacy stocking. Therefore, segmentation-driven strategies must align product design, evidence generation, and go-to-market channels to capture clinical relevance and operational efficiency simultaneously.
Comprehensive regional analysis revealing how distinct regulatory regimes, procurement practices, and clinical patterns in major geographies shape diuretics strategy and operations
Regional dynamics continue to shape strategic priorities as manufacturers and distributors align operations to local clinical practices, regulatory environments, and procurement models. The Americas region exhibits mature healthcare infrastructure and well-established reimbursement frameworks, which create an environment where formulary positioning, cost-effectiveness dossiers, and post-marketing safety surveillance are critical to commercial success. In contrast, Europe, Middle East & Africa presents a heterogeneous mix of single-payer systems, private payor markets, and emerging procurement entities, requiring adaptable market access strategies and localized clinical evidence generation to secure uptake across varied national settings. The Asia-Pacific region is characterized by rapid capacity expansion, diverse regulatory timelines, and a strong emphasis on cost-containment, prompting many organizations to pursue regional manufacturing partnerships, technology transfer arrangements, and tailored pricing models.
These regional distinctions have operational implications as well. Supply chain structures optimized for one geography may not translate efficiently to another, and regulatory dossiers often need substantive localization work to meet country-specific safety and quality requirements. Moreover, clinical practice patterns differ by region, influencing the relative demand for specific diuretic classes and dosage forms. Consequently, firms that invest in regionally nuanced market intelligence and flexible commercial playbooks are better positioned to navigate reimbursement negotiations, regulatory approvals, and distribution partnerships while sustaining supply continuity and meeting local clinician expectations.
Key corporate behaviors and strategic priorities emphasizing manufacturing resilience, evidence generation, and lifecycle management to sustain competitiveness in diuretics
Corporate approaches within the diuretics domain increasingly emphasize operational resilience, evidence generation, and pragmatic lifecycle management rather than relying solely on product innovation. Leading organizations are investing in manufacturing scale and redundancy to shorten lead times and reduce supplier concentration risk, while others focus on formulation enhancements and patient-centric delivery systems to protect margins in highly competitive generic markets. Strategic alliances and contract manufacturing partnerships remain common as firms balance capital intensity with the need for flexible production capacity.
On the clinical and commercial fronts, companies are strengthening value propositions through post-authorization studies, head-to-head safety comparisons, and targeted health economics work designed to resonate with payors and guidelines committees. These initiatives support formulary negotiations and help sustain product relevance in indications where therapeutic interchangeability is frequent. In addition, a subset of firms is integrating digital adherence and remote monitoring tools to enhance outcomes in chronic indications, thereby creating differentiated offerings that extend beyond the pill.
Finally, corporate governance across quality, regulatory affairs, and supply chain functions has gained prominence. Enhanced quality systems, proactive regulatory engagement, and scenario-based supply chain planning are emerging as baseline expectations for successful market participation, enabling companies to respond more quickly to inspections, policy shifts, and procurement disruptions while maintaining clinician and patient trust.
Actionable strategic recommendations for industry leaders to strengthen clinical evidence, fortify supply chains, and differentiate products for sustained commercial advantage
Industry leaders should take deliberate, measurable steps to align clinical evidence generation, manufacturing resiliency, and commercial execution with shifting stakeholder expectations. First, prioritize investment in robust real-world evidence and targeted comparative safety studies that directly address prescriber concerns about renal function and polypharmacy, thereby strengthening formulary positioning and payer negotiations. Align clinical programs to capture actionable endpoints and patient-reported outcomes that payors and guideline committees value, and integrate these data streams into commercial narratives.
Second, develop supply chain playbooks that include qualified secondary suppliers, regional manufacturing options, and strategic inventory policies to mitigate the impact of trade policy variability and logistics disruptions. Scenario planning and stress-testing of supplier networks will improve responsiveness and reduce the likelihood of acute shortages. Third, pursue differentiation through formulation optimization, fixed-dose combinations where clinically appropriate, and digital adherence tools that demonstrate measurable improvements in persistence and outcomes; these tactics help defend margins in price-sensitive markets.
Finally, engage proactively with regulators, procurement bodies, and large institutional buyers to clarify tariff classifications, secure potential exemptions where available, and co-design procurement pathways that reward demonstrated quality and reliability. By integrating these actions into a cohesive strategic plan, organizations can improve resilience, preserve clinical relevance, and create defensible commercial advantages in a competitive environment.
An evidence-driven research methodology combining rigorous secondary sources, targeted expert interviews, and triangulation protocols to produce defensible strategic insights
The research approach combined systematic secondary intelligence with targeted primary engagement to produce a rigorous, triangulated perspective on the diuretics landscape. Secondary analysis included peer-reviewed literature, regulatory guidance documents, clinical practice guidelines, and open-source manufacturing and trade policy reports to establish a factual baseline across clinical, regulatory, and supply chain dimensions. Primary research supplemented this foundation with structured interviews of clinicians, procurement officers, manufacturing experts, and payer representatives to surface practical insights about real-world use patterns, procurement constraints, and quality expectations.
Data synthesis followed a triangulation protocol to reconcile differences between published sources and practitioner perspectives, and to identify recurring themes that warranted deeper exploration. Quality assurance processes included source validation, cross-checking of regulatory citations, and methodological transparency to ensure that inferences were defensible and replicable. Limitations of the methodology were acknowledged, including variability in regional data availability and the dynamic nature of trade policy and regulatory changes; these caveats were addressed through scenario-based analysis rather than single-point projections.
Ethical considerations guided research conduct, with all primary participants engaged under informed consent and confidentiality protections. The resulting methodology provides a balanced, evidence-informed foundation for strategic decision-making while clearly delineating the assumptions and constraints that shaped the findings.
A conclusive synthesis emphasizing the enduring clinical role of diuretics coupled with the strategic imperatives of evidence, resilience, and stakeholder collaboration
In conclusion, the diuretics field stands at the intersection of enduring clinical utility and accelerating operational complexity. Clinical demand remains anchored in established indications, yet the pathway to commercial success is increasingly determined by a company’s ability to demonstrate differentiated clinical value, manage supply chain risk, and navigate evolving regulatory and procurement environments. Stakeholders across the value chain-manufacturers, clinicians, payors, and distributors-are converging on quality, resilience, and real-world outcomes as key determinants of sustained adoption.
Moving forward, organizations that combine targeted evidence generation with pragmatic supply chain and manufacturing strategies will be best positioned to respond to policy fluctuations and competitive pressures. Moreover, integrating patient-centric approaches such as improved formulations and digital support tools will help preserve therapeutic relevance amid cost pressures. Ultimately, a balanced emphasis on clinical credibility, operational agility, and stakeholder collaboration will define success in this mature yet evolving therapeutic category.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Diuretics Drugs Market
Companies Mentioned
The key companies profiled in this Diuretics Drugs market report include:- Aurobindo Pharma Limited
- Cipla Limited
- Dr. Reddy’s Laboratories Limited
- Fresenius Kabi AG
- Hikma Pharmaceuticals PLC
- Lupin Limited
- Sandoz International GmbH
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
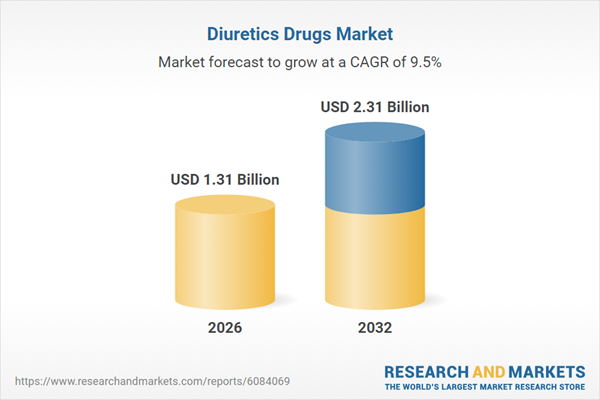
| Estimated Market Value ( USD | $ 1.31 Billion |
| Forecasted Market Value ( USD | $ 2.31 Billion |
| Compound Annual Growth Rate | 9.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |