Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative introduction framing how product choice, workflow compatibility, and supply resilience are reshaping specimen collection strategies across healthcare delivery
The landscape for bacterial and viral specimen collection has evolved from a predominantly reactive supply structure into a proactive, innovation-driven ecosystem. Advances in specimen types and collection technologies have pushed stakeholders to reassess procurement, logistics, and clinical protocols. At the same time, changing regulatory expectations and heightened demand for rapid, reliable diagnostics have elevated specimen integrity and traceability to strategic priorities.
Clinicians, laboratory managers, and procurement leaders now face an environment where product choice, end-user workflow compatibility, and distribution flexibility materially affect diagnostic outcomes. As a result, organizations must balance clinical performance with operational resilience, aligning product selection-such as swab type, transport media, and collection tube configuration-with laboratory automation and end-user needs. Looking ahead, this introductory synthesis frames the deeper insights that follow, emphasizing integration across product portfolios, technology adoption, and supply-chain agility as foundational to competitive positioning.
How technological innovation, regulatory evolution, and decentralization are jointly accelerating the evolution of specimen collection devices and workflows in healthcare delivery
Recent years have produced transformative shifts in specimen collection driven by converging forces: technology advancement, regulatory refinement, and the prioritization of continuity of care. Molecular diagnostics and genomic surveillance have created demand for sample types and transport media that preserve nucleic acid integrity over longer transit times, prompting shifts toward collection tubes and media optimized for downstream automated processing.
Concurrently, point-of-care testing expansion and decentralized diagnostics have elevated the importance of user-friendly collection devices. Swab innovation-ranging from flocked designs that improve specimen release to foam-based options for specific applications-has reduced collection variability. Automation has gained traction in high-throughput clinical laboratories, improving consistency and throughput while placing new requirements on tube dimensions and barcode compatibility. At the same time, digital traceability solutions have emerged to manage chain-of-custody and regulatory compliance, enabling faster turnarounds and improved data integrity. In response, manufacturers and distributors have repositioned portfolios to emphasize interoperability with automated platforms and robustness across diverse collection environments, signaling a durable, technology-led transformation in sample acquisition workflows.
Understanding how recent tariff policy adjustments have accelerated supplier diversification, domestic production strategies, and inventory resilience across diagnostic supply chains
The imposition of tariffs and trade policy adjustments has created ripple effects across procurement, manufacturing strategy, and product availability for specimen collection supplies. Tariff-driven cost pressures have incentivized buyers to reassess sourcing strategies, including seeking alternative suppliers, qualifying domestically manufactured components, and renegotiating long-term contracts to stabilize unit costs.
These shifts have encouraged manufacturers to re-evaluate their global footprint and to consider nearshoring or expanding local manufacturing capacity to mitigate the impact of cross-border duty changes. At the same time, distributors and end users have responded by diversifying supplier panels and increasing inventory buffers for critical items such as specialized swabs, collection tubes compatible with automated analyzers, and transport media that meet stringent preservation requirements. Regulatory compliance and quality assurance remain non-negotiable, and organizations balancing tariff-related cost management with these obligations have prioritized suppliers with validated quality systems and transparent documentation. Ultimately, the cumulative effect of tariff changes has accelerated strategic shifts toward supply-chain resilience, supplier qualification rigor, and manufacturing agility to ensure continuity of diagnostic services under evolving trade conditions.
Actionable segmentation analysis linking specimen types, product configurations, technology choices, end-user workflows, and distribution models to strategic decision-making
Segmentation drives strategic clarity by mapping product and user needs to clinical and operational contexts. Based on specimen type, the market distinguishes between bacterial and viral applications: bacterial collections often emphasize blood, urine, and wound specimens that demand transport media and devices preserving viable organisms for culture, while viral collections prioritize blood, nasopharyngeal swabs, and saliva specimens with an emphasis on nucleic acid preservation for molecular assays. This divergence in specimen requirements influences product choice and downstream laboratory workflows.
Based on product, decision-makers must account for the roles of collection kits, collection tubes, swabs, and transport media. Collection tubes present a choice between nonvacuum and vacuum designs, each affecting draw technique and compatibility with automated platforms. Swab selection ranges across cotton, flocked, and foam variants, with each material offering distinct collection and release characteristics that influence assay sensitivity. Transport media split into bacterial and viral formulations tailored to viability or nucleic acid stability, respectively, which has direct implications for storage and transport conditions. Based on technology, automation versus manual workflows determine throughput, reproducibility, and the needed physical and data interfaces between collection devices and laboratory analyzers. Based on end user, clinics, diagnostic centers, hospitals, and laboratories present different procurement behaviors and training needs, from low-volume, user-friendly kits for point-of-care settings to robust, automation-compatible consumables for high-volume labs. Finally, based on distribution channel, direct sales, distributors, and online sales each offer distinct service, pricing, and logistics models that shape lead times, volume discounts, and supplier relationships. Integrating these segmentation dimensions helps stakeholders prioritize investments, align product R&D with clinical needs, and design distribution strategies that match end-user expectations.
Regional dynamics that influence manufacturing strategy, regulatory navigation, and distribution priorities across Americas, Europe Middle East & Africa, and Asia-Pacific markets
Regional dynamics vary significantly and inform where companies should invest in manufacturing, regulatory support, and commercial outreach. In the Americas, procurement tends to prioritize supply reliability and regulatory compliance, with a strong emphasis on advanced diagnostics adoption and established distribution networks supporting hospitals and large reference laboratories. This region’s emphasis on rapid test turnaround and integration with health information systems encourages products that align with automated workflows and digital traceability.
In Europe, Middle East & Africa, regulatory landscapes and purchasing channels differ markedly across subregions, creating opportunities for suppliers who can navigate diverse registration requirements and offer flexible distribution arrangements. Demand in this broad region often reflects both established laboratory infrastructure in some markets and rapidly developing diagnostic capabilities in others, necessitating adaptable product configurations and robust technical support. In Asia-Pacific, a combination of manufacturing scale, innovation in point-of-care solutions, and heterogeneous healthcare systems drives both local production and export-oriented manufacturing strategies. Regional players in this area frequently focus on cost-effective production, scalability, and rapid iteration of swab and tube designs to meet varying clinical needs. Across all regions, logistics reliability, cold chain considerations for certain transport media, and regulatory alignment remain decisive factors shaping procurement and supply strategies.
How product innovation, validated manufacturing practices, and service-led partnerships are reshaping competitive advantage among specimen collection suppliers
Competitive dynamics reflect a mix of product innovation, supply-chain excellence, and strategic partnerships. Leading companies prioritize R&D investments that reduce collection variability and improve compatibility with automated laboratory workflows. Product differentiation often hinges on materials science for swab construction, tube engineering for sample stability, and formulation science for transport media optimized for either viability or nucleic acid preservation. Companies that combine reliable manufacturing, validated quality systems, and a responsive commercial footprint tend to secure long-term procurement relationships with hospital systems and large laboratories.
In addition, successful firms are expanding service layers such as training programs, digital tracking solutions, and customized kits that align with specific clinical pathways. Partnerships with clinical laboratories and integration with automation providers accelerate adoption by demonstrating real-world performance and reducing integration friction. Finally, companies that maintain flexible production capacity and transparent sourcing practices position themselves to respond rapidly to demand surges and regulatory changes, reinforcing their value proposition among procurement leaders focused on reliability and compliance.
Clear operational and commercial actions to build supply resilience, align products with automation, and deepen end-user partnerships to drive durable competitive advantage
Industry leaders should pursue a coordinated strategy that blends supply resilience, product differentiation, and end-user engagement. First, diversify sourcing by qualifying multiple suppliers for critical components and by evaluating nearshoring opportunities to reduce exposure to tariff volatility and transport delays. Concurrently, invest in manufacturing flexibility that allows rapid shifts between product types-such as swab formats or tube designs-to meet fluctuating clinical demand.
Second, accelerate integration with laboratory automation by ensuring product specifications, barcoding, and packaging align with high-throughput workflows, thereby reducing manual handling and improving throughput. Third, strengthen commercial offerings through education and technical support that demonstrate comparative performance across specimen types and collection protocols, helping clinicians and laboratory personnel reduce variability. Fourth, prioritize regulatory preparedness by maintaining comprehensive documentation and proactive engagement with regulatory agencies to expedite registrations and sustain market access. Finally, enhance distribution strategies by blending direct sales relationships for large institutional buyers with distributor partnerships and online channels for smaller facilities, ensuring both reach and responsive logistics. Taken together, these measures will help organizations secure durable demand and adapt to evolving clinical and trade environments.
A rigorous mixed-methods research approach combining stakeholder interviews, regulatory review, product evaluation, and triangulation to validate clinical and operational insights
This research synthesizes insights derived from a layered methodological approach that combines stakeholder engagement, product-level evaluation, and cross-functional validation. Primary data collection included structured interviews with laboratory directors, procurement managers, clinical end users, and supply-chain executives to capture firsthand perspectives on product performance, workflow compatibility, and sourcing priorities. These qualitative inputs were supplemented by systematic review of public regulatory filings, product descriptions, and technical dossiers to evaluate materials, formulations, and claims asserted by manufacturers.
To ensure rigor, triangulation methods reconciled interview findings with product specifications and procurement trends, and data cleansing protocols removed inconsistencies and ensured traceability. Scenario analysis explored the implications of supply-chain disruptions and policy changes, while usability assessment focused on swab ergonomics, tube handling, and compatibility with automated systems. Finally, findings underwent internal validation with subject-matter experts to confirm clinical relevance and operational feasibility, ensuring the conclusions presented are grounded in both practical experience and documented product characteristics.
A concise conclusion summarizing how product innovation, automation alignment, and supply resilience are central to future diagnostic reliability and strategic planning
In sum, the specimen collection landscape is at an inflection point where product choice, technological integration, and supply-chain strategy jointly determine diagnostic performance and operational resilience. Advances in swab design, transport media chemistry, and tube engineering pair with broader adoption of automation and digital traceability to reduce variability and accelerate result delivery. At the same time, policy shifts and trade dynamics have made supply diversification and manufacturing agility core strategic imperatives.
Stakeholders who align product portfolios with end-user workflows, invest in compatibility with automated platforms, and maintain rigorous supplier qualification will be best positioned to meet evolving clinical expectations. Continued collaboration across manufacturers, laboratories, and distribution partners will be essential to sustain innovation and ensure reliable access to high-quality specimens for both bacterial and viral diagnostics.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Bacterial & Viral Specimen Collection Market
Companies Mentioned
The key companies profiled in this Bacterial & Viral Specimen Collection market report include:- Avantor, Inc.
- Becton, Dickinson, and Company
- bioMérieux S.A.
- Cardinal Health
- Copan Group
- DiaSorin S.p.A.
- Dynarex Corporation
- Greiner Bio-One International GmbH
- Hardy Diagnostics
- HiMedia Laboratories Pvt. Ltd
- Longhorn Vaccines and Diagnostics, LLC
- Lucence Diagnostics Pte Ltd
- Medical Wire & Equipment
- Medline Industries, LP.
- Meridian Bioscience, Inc
- Miraclean Technology Co., Ltd
- Pretium Packaging
- Puritan Medical Products
- QuidelOrtho Corporation
- Stellar Scientific
- Thermo Fisher Scientific, Inc.
- Titan Biotech Ltd.
- Trinity Biotech PLC
- Vircell, S.L.
- Wuxi Nest Biotechnology Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
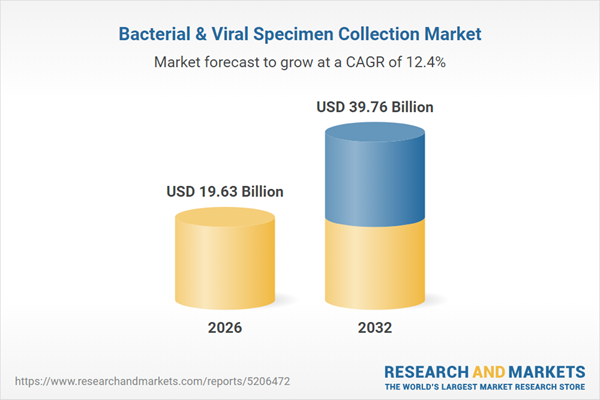
| Estimated Market Value ( USD | $ 19.63 Billion |
| Forecasted Market Value ( USD | $ 39.76 Billion |
| Compound Annual Growth Rate | 12.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |