Speak directly to the analyst to clarify any post sales queries you may have.
A strategic introduction framing clinical, commercial, and patient-driven imperatives reshaping alopecia treatment approaches and stakeholder priorities
Alopecia has transitioned from a primarily dermatological concern to a multidisciplinary strategic priority for healthcare providers, device manufacturers, biotechnology firms, and payers. Across clinical settings and consumer channels, patient expectations now emphasize efficacy, tolerability, convenience, and aesthetic outcomes, which together have reshaped care pathways and commercial models. Consequently, stakeholders must reconcile evolving clinical evidence with shifting reimbursement landscapes, digital care delivery, and heightened consumer awareness.As clinical innovation accelerates, the treatment environment is increasingly defined by the convergence of pharmacologic interventions, procedural approaches, and technology-enabled solutions. This convergence creates new opportunities for combination therapies, personalized regimens, and services that span prevention through restoration. At the same time, regulatory scrutiny and the demand for robust real-world evidence intensify, requiring companies to integrate outcomes measurement and patient-reported metrics into development plans and commercial narratives.
Taken together, these dynamics underscore the need for an integrated strategic perspective that aligns clinical development, commercialization, and stakeholder engagement. The following sections synthesize transformative shifts, policy influences, segmentation intelligence, regional nuances, competitive behaviors, and practical recommendations that collectively enable stakeholders to act with clarity and confidence.
Comprehensive overview of the scientific, technological, and care-delivery transformations that are redefining treatment pathways and patient journeys in alopecia care
The alopecia treatment landscape is undergoing transformative shifts driven by science, technology, and changing care models. On the scientific front, advances in understanding immunologic mechanisms, androgen signaling, and follicular biology have catalyzed a wave of targeted interventions and combination strategies. In parallel, minimally invasive procedural techniques and enhancements in hair transplantation logistics have elevated clinical outcomes and patient acceptance.Technological innovations are also redefining access and adherence. Digital diagnostics, teledermatology, and remote monitoring enable earlier intervention and more continuous care, while at-home devices and over-the-counter formulations expand the treatment continuum beyond clinic walls. These shifts are complemented by emerging biologics and regenerative approaches, which are prompting clinicians to reassess treatment sequences and to explore synergies between systemic agents, topical formulations, injectables, and device-based therapies.
Moreover, payer attitudes and regulatory frameworks are adapting to the interplay between aesthetic and medical indications, resulting in more nuanced coverage discussions. As a result, market participants must balance evidence generation with commercialization agility, emphasizing differentiated clinical benefit, patient experience, and scalable delivery models to capture long-term value in a fragmented and rapidly innovating market.
Analysis of how recent United States tariff adjustments have reshaped supply chain resilience, procurement strategies, and pricing dynamics in alopecia treatment delivery
Recent tariff policy changes in the United States introduced in 2025 have had a multifaceted effect on the supply chains and cost structures relevant to alopecia treatment stakeholders. Import duties and related trade measures have increased input costs for certain device components, biologics precursors, and specialized consumables that are often sourced internationally. As an immediate consequence, procurement teams have been prompted to reassess supplier diversification, inventory strategies, and landed-cost modeling to preserve margins and ensure continuity of care.In response, manufacturers and distributors have sought to optimize manufacturing footprints and to localize critical production stages where feasible. This reconfiguration has led to a heightened emphasis on supply chain resilience, including longer lead-time planning and strengthened contractual protections with strategic suppliers. Clinicians and specialty clinics have observed an incremental rise in procedure-related expenditure, which in turn has influenced patient out-of-pocket considerations and has catalyzed interest in value-based pricing conversations.
At the same time, the tariff environment has accelerated collaboration between device makers and domestic contract manufacturers, stimulated nearshoring discussions, and prompted payers to revisit coverage criteria where increased costs affect cost-effectiveness thresholds. Importantly, stakeholders are using this policy shift as an opportunity to invest in process innovation and to renegotiate supplier agreements, thereby converting a short-term cost pressure into an inflection point for long-term operational improvement.
In-depth segmentation insights across treatment modalities, condition subtypes, enabling technologies, demographic cohorts, and distribution channels to guide prioritization
Segmentation analysis reveals nuanced demand drivers and adoption patterns that are essential for prioritizing development and commercial efforts. When treatments are categorized by modality, Injectable Therapies encompass corticosteroid injections and platelet rich plasma, offering clinicians options for immunomodulation and regenerative stimulation respectively, while Laser Therapy spans hair growth devices and low level laser therapy which appeal to patients seeking noninvasive solutions. Oral Therapies include dutasteride, finasteride, and spironolactone, each addressing systemic pathways of hair loss and differing in risk-benefit profiles and patient suitability. Surgical Treatments cover hair transplantation and scalp micropigmentation, representing definitive restorative approaches that require specialized expertise and significant care coordination. Topical Therapies include corticosteroids and minoxidil, which remain foundational for many treatment algorithms due to ease of use and broad accessibility.Considering disease heterogeneity, the market differentiates across alopecia type: alopecia areata, with clinical presentations ranging from patchy to totalis and universalis, requires immune-targeted strategies and psychosocial support, whereas androgenetic alopecia separates into female and male patterns, each influenced by hormonal and genetic factors that guide therapeutic selection. Scarring alopecia, telogen effluvium, and traction alopecia present distinct etiologies and clinical management priorities, necessitating diagnostic precision and tailored interventions.
Technologically, the landscape includes low level laser therapy, platelet rich plasma, and stem cell therapy, with stem cell approaches subdivided into autologous hair follicle techniques and mesenchymal stem cell applications; these technologies vary by evidence maturity, regulatory pathway, and infrastructure needs. Age stratification into adolescent, adult, and geriatric cohorts further influences safety profiles, therapeutic preferences, and messaging. Distribution channel segmentation spans hospitals & clinics, online retail, pharmacies, and specialty clinics, each channel imposing different access mechanics, patient experience expectations, and commercial levers that companies must align with their product attributes and operational capabilities.
Regional intelligence highlighting how distinct regulatory, clinical, and commercial dynamics across the Americas, EMEA, and Asia-Pacific shape adoption and access
Regional dynamics materially influence clinical practice patterns, regulatory approaches, and commercial models in alopecia treatment, demanding region-specific strategies. In the Americas, clinical adoption is driven by a blend of advanced procedural expertise, private-pay clinics, and a consumer-oriented aesthetic market, which collectively support rapid uptake of both device-based therapies and surgical interventions. Regulatory clarity and payer heterogeneity in this region encourage companies to build strong evidence dossiers and to engage with specialty clinics and hospital systems for demonstration of value.Contrastingly, Europe, Middle East & Africa presents a mosaic of regulatory regimes and reimbursement frameworks, where clinical guidelines and public funding priorities vary considerably. In many EMEA markets, treatment adoption is influenced by public payer evaluations and regional centers of clinical excellence, which elevate the importance of health economic evidence and stakeholder engagement. Meanwhile, manufacturers often navigate disparate distribution architectures and cultural perceptions of aesthetic versus medical treatment, necessitating tailored messaging and local partnerships.
In the Asia-Pacific region, rapid urbanization, increasing healthcare spending, and growing consumer demand for minimally invasive and at-home treatments have accelerated interest in laser devices, topical regimens, and telemedicine-enabled care. However, regulatory pathways and clinical practice vary across jurisdictions, creating both opportunities for scale in populous markets and the need for phased market entry approaches that reflect local clinical preferences, supply chain realities, and distribution channel strengths.
Corporate and competitive insights emphasizing collaborations, clinical differentiation, and hybrid commercialization models that define leadership in alopecia care
Competitive behavior in the alopecia treatment ecosystem reflects a spectrum of strategic postures, ranging from vertically integrated device and biologics firms to specialized clinics and digital health entrants. Leading product developers increasingly emphasize clinical differentiation through robust safety and tolerability profiles, investment in head-to-head evidence, and targeted messaging that resonates with both clinicians and end users. At the same time, smaller innovative companies often focus on niche indications, regenerative approaches, or unique delivery platforms to secure strategic partnerships or licensing agreements with larger commercialization partners.Service providers and specialty clinics are building high-touch care models that blend procedural expertise with longitudinal patient support, loyalty programs, and bundled offerings. Digital-first entrants are leveraging teledermatology and direct-to-consumer channels to reduce friction in initial consultations and to drive product trial, particularly for topical and at-home device categories. Across the competitive landscape, collaboration is a recurrent theme: partnerships between biopharma and device manufacturers, alliances with contract manufacturing organizations, and co-development arrangements with clinical networks are increasingly used to accelerate market entry and to distribute risk.
As a result, companies that balance clinical credibility, supply chain robustness, and scalable distribution will be better positioned to capture demand across the spectrum from preventive care through restorative interventions. Importantly, leadership is earned not only through innovation but by demonstrable outcomes, patient experience, and operational excellence.
Practical and actionable strategic recommendations for manufacturers, clinicians, and service providers to build resilient, patient-focused, and scalable alopecia care models
Industry leaders should adopt a pragmatic, multi-dimensional strategy that aligns scientific differentiation with commercial execution and patient-centric care. First, invest in evidence generation that addresses comparative effectiveness and long-term safety across modality combinations, while integrating patient-reported outcomes to strengthen the value proposition for clinicians and payers. Second, prioritize supply chain resilience by diversifying suppliers, exploring regional manufacturing options, and embedding flexibility into procurement and inventory practices to mitigate tariff and logistical risks.Concurrently, stakeholders must refine channel strategies: align premium procedural offerings with specialty clinics and hospitals, while optimizing digital platforms, subscription models, and pharmacy partnerships to expand reach for at-home and topical therapies. Embrace technology-enabled care by integrating telehealth, remote monitoring, and data capture to improve adherence, enable early intervention, and generate real-world evidence. Additionally, cultivate strategic collaborations across biopharma, device makers, and service providers to combine complementary capabilities and accelerate clinical validation.
Finally, enhance stakeholder engagement by proactively addressing reimbursement and regulatory questions, investing in local market education, and developing flexible pricing models that reflect clinical value and patient affordability. By executing these actions in concert, organizations can convert complexity into competitive advantage and deliver sustained improvements in patient outcomes.
Methodological overview describing the triangulated research approach, primary interviews, literature synthesis, and validation processes used to ensure robust insights
This research synthesizes qualitative and quantitative evidence gathered through a triangulated methodology that emphasizes transparency and reproducibility. Primary inputs included structured interviews with dermatologists, proceduralists, specialty clinic operators, payers, and patient advocates to capture frontline perspectives on clinical decision-making, adoption barriers, and unmet needs. Secondary inputs entailed a systematic review of peer-reviewed clinical literature, regulatory guidance documents, and relevant health technology assessments to ground insights in the latest clinical and policy evidence.Analytical techniques combined thematic analysis of stakeholder interviews with cross-sectional mapping of technology readiness, clinical evidence maturity, and channel economics. Sensitivity analyses explored key operational variables such as procurement exposure, device component sourcing, and distribution channel mixes to understand resilience under differing supply chain scenarios. Throughout the process, findings were validated via expert advisory panels to ensure interpretive rigor and to surface practical implications for commercialization and clinical practice.
Confidentiality safeguards and data-verification protocols were applied to primary data, and limitations are acknowledged where evidence gaps exist, particularly for nascent regenerative therapies and emerging device platforms. Where appropriate, recommendations are framed to accommodate variability in regulatory frameworks and payer environments across jurisdictions.
Synthesis and concluding perspectives that integrate clinical, commercial, and operational imperatives to inform strategic decision-making in alopecia care
In conclusion, the alopecia treatment landscape is at an inflection point where scientific advances, technology adoption, and shifting payer and patient expectations intersect to create both opportunity and complexity. Stakeholders that pursue integrated strategies-combining differentiated clinical evidence, resilient operations, and channel-tailored commercialization-will be better positioned to translate innovation into meaningful patient outcomes. Furthermore, policy shifts and supply chain volatility underscore the importance of operational agility and strategic partnerships.Looking ahead, success will depend on aligning product development with real-world needs, demonstrating sustained clinical benefit, and delivering care in formats that reflect modern patient preferences. By emphasizing evidence-based differentiation, investing in end-to-end delivery capabilities, and proactively engaging payers and clinicians, organizations can navigate current headwinds and build durable value propositions across the continuum of alopecia care.
These conclusions should serve as the foundation for prioritized investment, partnership selection, and market entry sequencing, enabling leaders to act decisively while remaining adaptive to evolving clinical and commercial signals.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Alopecia Treatment Market
Companies Mentioned
The key companies profiled in this Alopecia Treatment market report include:- AbbVie Inc.
- Aclaris Therapeutics, Inc.
- AnaptysBio Inc.
- Apira Science, Inc.
- Apple Therapeutics Private Limited
- Arcutis Biotherapeutics, Inc.
- Biocon Limited
- Cipla Limited
- Daiichi Sankyo Inc.
- DR. KURT WOLFF GMBH & CO. KG
- Dr. Reddy’s Laboratories Ltd.
- Eli Lilly and Company
- Fagron NV
- Follica Inc.
- GlaxoSmithKline plc
- HCell Inc.
- Histogen Inc.
- Intas Pharmaceuticals Ltd.
- iRestore Hair Growth System
- Johnson & Johnson Services, Inc.
- KNOW Bio LLC
- Merck & Co., Inc.
- Pfizer Inc.
- Regeneron Pharmaceuticals, Inc.
- RepliCel Life Sciences Inc.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Transitions Hair Pty Ltd.
- Viatris Inc.
- Viviscal
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
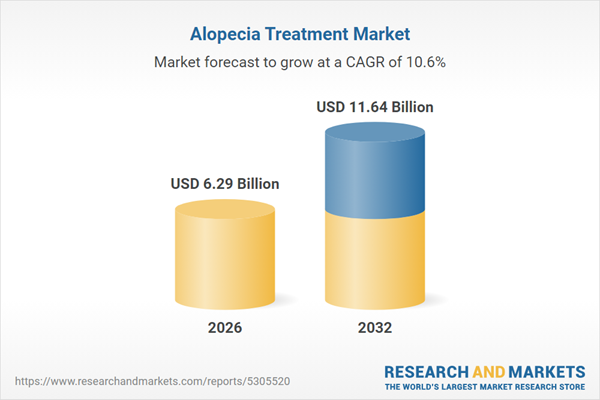
| Estimated Market Value ( USD | $ 6.29 Billion |
| Forecasted Market Value ( USD | $ 11.64 Billion |
| Compound Annual Growth Rate | 10.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 31 |