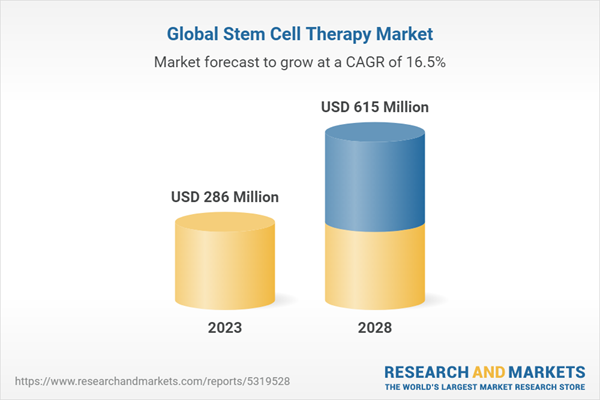
1 Introduction
1.1 Study Objectives
1.2 Market Definition
1.3 Inclusions & Exclusions
1.4 Market Scope
1.4.1 Markets Covered
1.4.2 Years Considered
1.5 Currency
1.6 Research Limitations
1.7 Stakeholders
1.8 Summary of Changes
1.9 Recession Impact
2 Research Methodology
2.1 Research Data
Figure 1 Research Design
2.1.1 Secondary Data
2.1.2 Primary Data
Figure 2 Breakdown of Primaries: Stem Cell Therapy Market
2.2 Market Size Estimation
Figure 3 Market Size Estimation for Supply-Side Analysis, 2022
Figure 4 Market Size Estimation: Revenue Share Analysis, 2022
Figure 5 Smith+Nephew: Revenue Share Analysis, 2022
2.2.1 Primary Insights
Figure 6 Validation from Primary Experts
2.2.2 Segment Assessment Methodology: Stem Cell Therapy Industry
Figure 7 Market Size Estimation Methodology: Top-Down Approach
Figure 8 CAGR Projections: Market
Figure 9 Growth Analysis of Drivers, Restraints, Challenges, and Opportunities
2.3 Market Breakdown and Data Triangulation
Figure 10 Data Triangulation Methodology
2.4 Study Assumptions
2.5 Risk Analysis
2.6 Impact of Recession on Market
Table 1 Global Inflation Rate Projection, 2024-2028 (% Growth)
Table 2 US Health Expenditure, 2019-2022 (USD Million)
Table 3 US Health Expenditure, 2023-2027 (USD Million)
3 Executive Summary
Figure 11 Stem Cell Therapy Market, by Type, 2023 vs. 2028 (USD Million)
Figure 12 Market, by Cell Source, 2023 vs. 2028 (USD Million)
Figure 13 Market, by Therapeutic Application, 2023 vs. 2028 (USD Million)
Figure 14 Regional Snapshot of Market
4 Premium Insights
4.1 Market Overview
Figure 15 Increasing Investments and Funding for Stem Cell Research to Drive Market
4.2 North America: Market, by Type and Country, 2022
Figure 16 Allogeneic Stem Cell Therapy Accounted for Largest Market Share in 2022
4.3 Stem Cell Therapy Industry: Geographical Growth Opportunities
Figure 17 South Korea to Register Highest Growth Rate During Forecast Period
5 Market Overview
5.1 Introduction
5.2 Market Dynamics
Figure 18 Stem Cell Therapy Market: Drivers, Restraints, Opportunities, and Challenges
5.2.1 Drivers
5.2.1.1 Increased Funding for Stem Cell Research
Table 4 Funding for Stem Cell Research by Indian Council of Medical Research, 2019-2022 (USD)
Table 5 Funding for Cell-based Research by National Institutes of Health, 2018-2023 (USD Million)
5.2.1.2 Rising Number of Collaborations Among Healthcare Institutes
5.2.1.3 Increasing Number of Clinical Trials for Stem Cell-based Therapies
Figure 19 Number of Clinical Trials, 2015-2022
5.2.2 Restraints
5.2.2.1 Ethical Concerns Related to Use of Embryonic Stem Cells
5.2.2.2 High Cost of Cell-based Research and Stem Cell Therapy
5.2.3 Opportunities
5.2.3.1 Increased Availability of Alternatives to Embryonic Stem Cells
5.2.3.2 Growing Demand for Cell and Gene Therapies
5.2.4 Challenges
5.2.4.1 Technological Limitations
5.3 Technology Analysis
Table 6 Comparison Between Stem Cell Therapies and Gene Therapies
5.4 Disruptions and Trends Impacting Customer's Business
Figure 20 Revenue Shift and New Pocket for Key Players in the Market
5.5 Value Chain Analysis
Figure 21 Stem Cell Therapy Industry: Value Chain Analysis
5.6 Ecosystem Market Map
Figure 22 Market: Ecosystem Market Map
5.7 Supply Chain Analysis
Figure 23 Market: Supply Chain Analysis
Table 7 Supply Chain Analysis: Role of Commercial-Scale/Key Manufacturers
Table 8 Supply Chain Analysis: Pipeline/Emerging Manufacturers
5.8 Porter's Five Forces Analysis
5.8.1 Threat of New Entrants
5.8.2 Threat of Substitutes
5.8.3 Bargaining Power of Suppliers
5.8.4 Bargaining Power of Buyers
5.8.5 Intensity of Competition Rivalry
5.9 Regulatory Analysis
5.9.1 Regulatory Landscape
5.9.1.1 North America
Table 9 North America: Regulatory Landscape for Stem Cell Therapies
5.9.1.2 Europe
Table 10 Europe: Regulatory Landscape for Stem Cell Therapies
5.9.1.3 Asia-Pacific
Table 11 Asia-Pacific: Regulatory Landscape for Stem Cell Therapies
5.9.1.4 Rest of the World
Table 12 Rest of the World: Regulatory Landscape for Stem Cell Therapies
5.9.2 Regulatory Bodies, Government Agencies, and Other Organizations
Table 13 North America: Regulatory Bodies, Government Agencies, and Other Organizations
Table 14 Europe: Regulatory Bodies, Government Agencies, and Other Organizations
Table 15 Asia-Pacific: Regulatory Bodies, Government Agencies, and Other Organizations
Table 16 Rest of the World: Regulatory Bodies, Government Agencies, and Other Organizations
5.10 Pricing Analysis
5.10.1 Pricing Analysis
Table 17 Average Selling Price of Drug Therapies Offered by Key Players in the Market
5.10.2 Average Selling Price Trend for Stem Cell Therapies
5.11 Patent Analysis
Figure 24 Stem Cell Therapy: Patent Analysis, 2013-2023
5.12 Key Conferences & Events
Table 18 List of Key Conferences & Events, 2023-2024
5.13 Pipeline Analysis
5.14 Key Stakeholders & Buying Criteria
Figure 25 Key Stakeholders
5.14.1 Key Buying Criteria
Figure 26 Key Buying Criteria for End-users
6 Stem Cell Therapy Market, by Cell Source
6.1 Introduction
Table 19 Stem Cell Therapy Industry, by Cell Source, 2021-2028 (USD Million)
6.2 Adipose Tissue-Derived Mesenchymal Stem Cells
6.2.1 Ease of Isolation and Harvesting to Drive Growth
Table 20 Adipose Tissue-Derived Mesenchymal Stem Cells Market, by Region, 2021-2028 (USD Million)
Table 21 North America: Adipose Tissue-Derived Mesenchymal Stem Cells Market, by Country, 2021-2028 (USD Million)
Table 22 Europe: Adipose Tissue-Derived Mesenchymal Stem Cells Market, by Country, 2021-2028 (USD Million)
Table 23 Asia-Pacific: Adipose Tissue-Derived Mesenchymal Stem Cells Market, by Country, 2021-2028 (USD Million)
6.3 Bone Marrow-Derived Mesenchymal Stem Cells
6.3.1 High Prevalence of Metabolic Disorders to Support Market Growth
Table 24 Bone Marrow-Derived Mesenchymal Stem Cells Market, by Region, 2021-2028 (USD Million)
Table 25 North America: Bone Marrow-Derived Mesenchymal Stem Cells Market, by Country, 2021-2028 (USD Million)
Table 26 Europe: Bone Marrow-Derived Mesenchymal Stem Cells Market, by Country, 2021-2028 (USD Million)
Table 27 Asia-Pacific: Bone Marrow-Derived Mesenchymal Stem Cells Market, by Country, 2021-2028 (USD Million)
6.4 Placenta/Umbilical Cord-Derived Mesenchymal Stem Cells
6.4.1 Low Chances of Rejection from Immune System to Propel Growth
Table 28 Placenta/Umbilical Cord-Derived Mesenchymal Stem Cells Market, by Region, 2021-2028 (USD Million)
Table 29 North America: Placenta/Umbilical Cord-Derived Mesenchymal Stem Cells Market, by Country, 2021-2028 (USD Million)
Table 30 Europe: Placenta/Umbilical Cord-Derived Mesenchymal Stem Cells Market, by Country, 2021-2028 (USD Million)
Table 31 Asia-Pacific: Placenta/Umbilical Cord-Derived Mesenchymal Stem Cells Market, by Country, 2021-2028 (USD Million)
6.5 Other Cell Sources
Table 32 Market for Other Cell Sources, by Region, 2021-2028 (USD Million)
Table 33 North America: Market for Other Cell Sources, by Country, 2021-2028 (USD Million)
Table 34 Europe: Market for Other Cell Sources, by Country, 2021-2028 (USD Million)
Table 35 Asia-Pacific: Market for Other Cell Sources, by Country, 2021-2028 (USD Million)
7 Stem Cell Therapy Market, by Type
7.1 Introduction
Table 36 Stem Cell Therapy Industry, by Type, 2021-2028 (USD Million)
7.2 Allogeneic Stem Cell Therapy
7.2.1 Economically Viable and Less Time-Consuming - Key Factors Driving Market Growth
Table 37 Market, by Region, 2021-2028 (USD Million)
Table 38 North America: Market, by Country, 2021-2028 (USD Million)
Table 39 Europe: Market, by Country, 2021-2028 (USD Million)
Table 40 Asia-Pacific: Market, by Country, 2021-2028 (USD Million)
7.3 Autologous Stem Cell Therapy
7.3.1 Low Risk of Post-Treatment Complications to Drive Growth
Table 41 Market, by Region, 2021-2028 (USD Million)
Table 42 North America: Market, by Country, 2021-2028 (USD Million)
Table 43 Europe: Market, by Country, 2021-2028 (USD Million)
Table 44 Asia-Pacific: Market, by Country, 2021-2028 (USD Million)
8 Stem Cell Therapy Market, by Therapeutic Application
8.1 Introduction
Table 45 Stem Cell Therapy Industry, by Therapeutic Application, 2021-2028 (USD Million)
8.2 Musculoskeletal Disorders
8.2.1 Increasing Cases of Osteoarthritis to Drive Market
Table 46 Market for Musculoskeletal Disorders, by Region, 2021-2028 (USD Million)
Table 47 North America: Market for Musculoskeletal Disorders, by Country, 2021-2028 (USD Million)
Table 48 Europe: Market for Musculoskeletal Disorders, by Country, 2021-2028 (USD Million)
Table 49 Asia-Pacific: Market for Musculoskeletal Disorders, by Country, 2021-2028 (USD Million)
8.3 Wounds & Surgeries
8.3.1 Increasing Benefits of Allogeneic-based Therapies to Support Market Growth
Table 50 Market for Wounds & Surgeries, by Region, 2021-2028 (USD Million)
Table 51 North America: Market for Wounds & Surgeries, by Country, 2021-2028 (USD Million)
Table 52 Europe: Market for Wounds & Surgeries, by Country, 2021-2028 (USD Million)
Table 53 Asia-Pacific: Market for Wounds & Surgeries, by Country, 2021-2028 (USD Million)
8.4 Inflammatory & Autoimmune Diseases
8.4.1 Increasing Clinical Trials to Support Market Growth
Table 54 Market for Inflammatory & Autoimmune Diseases, by Region, 2021-2028 (USD Million)
Table 55 North America: Market for Inflammatory & Autoimmune Diseases, by Country, 2021-2028 (USD Million)
Table 56 Europe: Market for Inflammatory & Autoimmune Diseases, by Country, 2021-2028 (USD Million)
Table 57 Asia-Pacific: Market for Inflammatory & Autoimmune Diseases, by Country, 2021-2028 (USD Million)
8.5 Cardiovascular Diseases
8.5.1 Increasing Public & Private Funding for Cvd Research to Drive Market
Table 58 Market for Cardiovascular Diseases, by Region, 2021-2028 (USD Million)
Table 59 North America: Market for Cardiovascular Diseases, by Country, 2021-2028 (USD Million)
Table 60 Europe: Market for Cardiovascular Diseases, by Country, 2021-2028 (USD Million)
Table 61 Asia-Pacific: Market for Cardiovascular Diseases, by Country, 2021-2028 (USD Million)
8.6 Neurological Disorders
8.6.1 Rising Prevalence of Neurological Disorders to Propel Market
Table 62 Market for Neurological Disorders, by Region, 2021-2028 (USD Million)
Table 63 North America: Market for Neurological Disorders, by Country, 2021-2028 (USD Million)
Table 64 Europe: Market for Neurological Disorders, by Country, 2021-2028 (USD Million)
Table 65 Asia-Pacific: Market for Neurological Disorders, by Country, 2021-2028 (USD Million)
8.7 Other Therapeutic Applications
Table 66 Market for Other Therapeutic Applications, by Region, 2021-2028 (USD Million)
Table 67 North America: Market for Other Therapeutic Applications, by Country, 2021-2028 (USD Million)
Table 68 Europe: Market for Other Therapeutic Applications, by Country, 2021-2028 (USD Million)
Table 69 Asia-Pacific: Market for Other Therapeutic Applications, by Country, 2021-2028 (USD Million)
9 Stem Cell Therapy Market, by Region
9.1 Introduction
Table 70 Stem Cell Therapy Industry, by Region, 2021-2028 (USD Million)
9.2 North America
Figure 27 North America: Market Snapshot
Table 71 North America: Market, by Country, 2021-2028 (USD Million)
Table 72 North America: Market, by Cell Source, 2021-2028 (USD Million)
Table 73 North America: Market, by Type, 2021-2028 (USD Million)
Table 74 North America: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.2.1 US
9.2.1.1 Increase in Stem Cell Therapy Approvals to Drive Market
Table 75 US: Stem Cell Therapy Market, by Cell Source, 2021-2028 (USD Million)
Table 76 US: Market, by Type, 2021-2028 (USD Million)
Table 77 US: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.2.2 Canada
9.2.2.1 Increase in Research Activities Targeted Toward Stem Cell Therapies to Propel Market
Table 78 Canada: Market, by Cell Source, 2021-2028 (USD Million)
Table 79 Canada: Market, by Type, 2021-2028 (USD Million)
Table 80 Canada: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.2.3 Recession Impact on North American Market
9.3 Europe
Table 81 Europe: Stem Cell Therapy Market, by Country, 2021-2028 (USD Million)
Table 82 Europe: Market, by Cell Source, 2021-2028 (USD Million)
Table 83 Europe: Market, by Type, 2021-2028 (USD Million)
Table 84 Europe: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.3.1 Germany
9.3.1.1 Increasing Incidence of Sports-Related Injuries to Drive Market
Table 85 Germany: Market, by Cell Source, 2021-2028 (USD Million)
Table 86 Germany: Market, by Type, 2021-2028 (USD Million)
Table 87 Germany: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.3.2 UK
9.3.2.1 Rising Adoption of Cell-based Therapies to Propel Market
Table 88 UK: Stem Cell Therapy Market, by Cell Source, 2021-2028 (USD Million)
Table 89 UK: Market, by Type, 2021-2028 (USD Million)
Table 90 UK: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.3.3 France
9.3.3.1 Rising R&D Expenditure for Stem Cell Therapy to Support Market Growth
Table 91 France: Market, by Cell Source, 2021-2028 (USD Million)
Table 92 France: Market, by Type, 2021-2028 (USD Million)
Table 93 France: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.3.4 Italy
9.3.4.1 Rising Prevalence of Neurological and Cardiovascular Disorders to Drive Market
Table 94 Italy: Stem Cell Therapy Market, by Cell Source, 2021-2028 (USD Million)
Table 95 Italy: Market, by Type, 2021-2028 (USD Million)
Table 96 Italy: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.3.5 Spain
9.3.5.1 Rising Prevalence of Cvd to Propel Market
Table 97 Spain: Market, by Cell Source, 2021-2028 (USD Million)
Table 98 Spain: Market, by Type, 2021-2028 (USD Million)
Table 99 Spain: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.3.6 Rest of Europe
Table 100 Rest of Europe: Stem Cell Therapy Market, by Cell Source, 2021-2028 (USD Million)
Table 101 Rest of Europe: Market, by Type, 2021-2028 (USD Million)
Table 102 Rest of Europe: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.3.7 Recession Impact on European Market
9.4 Asia-Pacific
Figure 28 Asia-Pacific: Market Snapshot
Table 103 Asia-Pacific: Market, by Country, 2021-2028 (USD Million)
Table 104 Asia-Pacific: Market, by Cell Source, 2021-2028 (USD Million)
Table 105 Asia-Pacific: Market, by Type, 2021-2028 (USD Million)
Table 106 Asia-Pacific: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.4.1 Japan
9.4.1.1 Growing Geriatric Population and Faster Regulatory Approval Process to Augment Market Growth
Table 107 Japan: Stem Cell Therapy Market, by Cell Source, 2021-2028 (USD Million)
Table 108 Japan: Market, by Type, 2021-2028 (USD Million)
Table 109 Japan: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.4.2 South Korea
9.4.2.1 Presence of Major Players to Support Market Growth in South Korea
Table 110 South Korea: Market, by Cell Source, 2021-2028 (USD Million)
Table 111 South Korea: Market, by Type, 2021-2028 (USD Million)
Table 112 South Korea: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.4.3 India
9.4.3.1 Rising Incidence of Neurodegenerative Disorders and Diabetes to Drive Market
Table 113 India: Stem Cell Therapy Market, by Cell Source, 2021-2028 (USD Million)
Table 114 India: Market, by Type, 2021-2028 (USD Million)
Table 115 India: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.4.4 China
9.4.4.1 Increasing Investments in Stem Cell Research to Drive Market
Table 116 China: Market, by Cell Source, 2021-2028 (USD Million)
Table 117 China: Market, by Type, 2021-2028 (USD Million)
Table 118 China: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.4.5 Rest of Asia-Pacific
Table 119 Rest of Asia-Pacific: Stem Cell Therapy Market, by Cell Source, 2021-2028 (USD Million)
Table 120 Rest of Asia-Pacific: Market, by Type, 2021-2028 (USD Million)
Table 121 Rest of Asia-Pacific: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.4.6 Recession Impact on Asia-Pacific Market
9.5 Rest of the World
Table 122 Rest of the World: Market, by Cell Source, 2021-2028 (USD Million)
Table 123 Rest of the World: Market, by Type, 2021-2028 (USD Million)
Table 124 Rest of the World: Market, by Therapeutic Application, 2021-2028 (USD Million)
9.5.1 Recession Impact on Rest of the World Market
10 Competitive Landscape
10.1 Introduction
10.2 Strategies Adopted by Key Players
Figure 29 Stem Cell Therapy Market: Strategies Adopted by Key Players
10.3 Revenue Share Analysis
Figure 30 Revenue Analysis of Top Players, 2020-2022 (USD Million)
10.4 Market Share Analysis
Figure 31 Stem Cell Therapy Market: Market Share Analysis, by Key Player, 2022
Table 125 Stem Cell Therapy Industry: Intensity of Competitive Rivalry
10.5 Company Evaluation Matrix
Figure 32 Stem Cell Therapy Industry: Company Evaluation Matrix for Key Players, 2022
10.5.1 Stars
10.5.2 Emerging Leaders
10.5.3 Pervasive Players
10.5.4 Participants
10.6 Competitive Benchmarking of Key Players
Table 126 Therapeutic Application Footprint of Companies
10.7 Regional Footprint of Top 15 Companies
Table 127 Regional Footprint of Companies
10.8 Startup/SME Evaluation Matrix
Figure 33 Stem Cell Therapy Market: Startup/SME Evaluation Matrix, 2022
10.8.1 Progressive Companies
10.8.2 Responsive Companies
10.8.3 Dynamic Companies
10.8.4 Starting Blocks
10.9 Competitive Benchmarking of Startups/SMEs
Table 128 Stem Cell Therapy Industry: Product Footprint Analysis of Startups/SMEs
Table 129 Stem Cell Therapy Industry: Competitive Benchmarking of Startups/SMEs
10.10 Competitive Scenarios and Trends
10.10.1 Product Approvals
Table 130 Stem Cell Therapy Market: Product Approvals, January 2020-October 2023
10.10.2 Deals
Table 131 Stem Cell Therapy Market: Deals, January 2020-October 2023
10.10.3 Other Developments
Table 132 Stem Cell Therapy Industry: Other Developments, January 2020-October 2023
11 Company Profiles
Business Overview, Products Offered, Recent Developments, and Analyst's View (Key Strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats)
11.1 Key Players
11.1.1 Smith+Nephew
Table 133 Smith+Nephew: Business Overview
Figure 34 Smith+Nephew: Company Snapshot (2022)
11.1.2 Medipost
Table 134 Medipost: Business Overview
Figure 35 Medipost: Company Snapshot (2021)
11.1.3 JCR Pharmaceuticals Co. Ltd.
Table 135 Jcr Pharmaceuticals Co. Ltd.: Business Overview
Figure 36 Jcr Pharmaceuticals Co. Ltd.: Company Snapshot (2022)
11.1.4 Takeda Pharmaceutical Company Limited
Table 136 Takeda Pharmaceutical Company Limited: Business Overview
Figure 37 Takeda Pharmaceutical Company Limited: Company Snapshot (2022)
11.1.5 Anterogen Co. Ltd.
Table 137 Anterogen Co. Ltd.: Business Overview
11.1.6 Corestem, Inc.
Table 138 Corestem Inc.: Business Overview
Figure 38 Corestem Inc.: Company Snapshot (2021)
11.1.7 Pharmicell Co. Ltd.
Table 139 Pharmicell Co. Ltd.: Business Overview
11.1.8 Nuvasive, Inc.
Table 140 Nuvasive, Inc.: Business Overview
Figure 39 Nuvasive, Inc.: Company Snapshot (2022)
11.1.9 Rti Surgical
Table 141 Rti Surgical: Business Overview
11.1.10 Allosource
Table 142 Allosource: Business Overview
11.1.11 Holostem Terapie Avanzate Srl
Table 143 Holostem Terapie Avanzate Srl: Business Overview
11.1.12 Orthofix Medical Inc.
Table 144 Orthofix Medical Inc.: Business Overview
Figure 40 Orthofix Medical Inc.: Company Snapshot (2022)
11.1.13 Stempeutics Research Pvt Ltd.
Table 145 Stempeutics Research Pvt Ltd.: Business Overview
11.1.14 Regrow Biosciences Pvt Ltd.
Table 146 Regrow Biosciences Pvt Ltd.: Business Overview
11.2 Other Players
11.2.1 Athersys, Inc.
Table 147 Athersys, Inc.: Business Overview
11.2.2 Mesoblast Ltd.
Table 148 Mesoblast Ltd.: Business Overview
11.2.3 Biorestorative Therapies, Inc.
Table 149 Biorestorative Therapies, Inc.: Business Overview
11.2.4 Pluristem Therapeutics Inc.
Table 150 Pluristem Therapeutics Inc.: Business Overview
11.2.5 Brainstorm Cell Limited
Table 151 Brainstorm Cell Limited: Business Overview
11.2.6 Gamida Cell
Table 152 Gamida Cell: Business Overview
11.2.7 Viacyte, Inc.
Table 153 Viacyte, Inc.: Business Overview
11.2.8 Kangstem Biotech Co. Ltd.
Table 154 Kangstem Biotech Co. Ltd.: Business Overview
11.2.9 Hope Biosciences
Table 155 Hope Biosciences: Business Overview
11.2.10 Cellular Biomedicine Group
Table 156 Cellular Biomedicine Group: Business Overview
11.2.11 Personalized Stem Cells
Table 157 Personalized Stem Cells: Business Overview
Details on Business Overview, Products Offered, Recent Developments, and Analyst's View (Key Strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats) Might Not be Captured in Case of Unlisted Companies
12 Appendix
12.1 Discussion Guide
12.2 Knowledgestore: The Subscription Portal
12.3 Customization Options