Integration of IVD Technologies with Digital Health Solutions Fuels South & Central America In-Vitro Diagnostics Market
IVD is used in clinical, laboratory, and outpatient settings with the aim specifically to help in the detection of diseases and, consequently, aid in the selection of appropriate treatment protocols. The integration of IVD technologies with digital health solutions is gaining traction globally. Data analytics, artificial Intelligence, and remote monitoring enhance the value of diagnostic tests, leading to better patient management and outcomes. IVD technologies integrated with digital health solutions can be incorporated into clinical decision support systems. As recognized by the WHO, digital health solutions could help detect diseases. Artificial intelligence health bots and similar other emerging solutions may present opportunities for patient care and address challenges such as high cost and time requirements. In diagnostics based on genomic testing, deep learning can identify cancer cells, determine their type, and predict what mutations may occur in a tumor from images of a specific sample. Artificial intelligence and machine learning (AI/ML) in in-vitro diagnostics are revolutionizing medical device development. These modern diagnostic systems facilitate diagnosis based on digital image analysis, thereby improving healthcare decision-making. Smart diagnostics are extremely scalable IVD solutions that use artificial intelligence to perform better than lab-based diagnostics at a fraction of the price. Additionally, this type of diagnostics can derive emergent features through unique chemical and biological signature detection and analysis. Thus, the integration of IVD with digital health technologies is likely to offer lucrative opportunities to the in-vitro diagnostics market in the coming years.South & Central America In-Vitro Diagnostics Market Overview
The South & Central America in-vitro diagnostics market has been segmented into Brazil, Argentina, and the Rest of South & Central America. The market for in-vitro diagnostics in the region is expected to grow during the forecast period owing to the higher prevalence of chronic conditions, increasing number of diagnostic facilities, and developing healthcare infrastructure. The aforementioned factors are responsible for influential growth of in-vitro diagnostics market in the South & Central America region. Brazil is the largest market in South & Central America. Factors such as supportive government, lucrative healthcare policies, a developing economy, and advancing healthcare infrastructure create scope for adopting modern technologies in diagnostics. Additionally, the increasing prevalence of chronic conditions and infectious diseases is projected to drive the growth of the in-vitro diagnostics market in the country. According to a study published by the International Diabetes Federation, the diabetic population of the country is projected to reach 21.8 million by 2045 from 15.7 million in 2021. Also, according to the estimates, Brazil has the fifth largest diabetic population in the world. A number of major players in the in-vitro diagnostics market take strategic initiatives to consolidate their market through geographic expansions in Brazil. For instance, in August 2022, Fresenius Kabi partnered with Bio-Manguinhos/Fiocruz and Bionovis to provide access to adalimumab biosimilar in Brazil. This partnership will expand treatment options for several autoimmune diseases in the Brazilian Public Health System (SUS).Additionally, Brazil is witnessing a high rate of growth in the geriatric population. For instance, according to the Pan American Health Organization, Brazil has more than 30 million people aged 60 years or more, which represents 13% of the country's population. The use of molecular diagnostic techniques in developing and, subsequently, administering personalized medicine is likely to boost the market growth in Brazil. On February 28, 2020, Cepheid and Sherlock Biosciences collaborated to explore the development of new cutting-edge molecular diagnostic tests. The collaboration will focus on the development of molecular diagnostic tests for oncological conditions and infectious diseases. Brazil is the largest healthcare market in Latin America, spending 9.1% of its GDP on healthcare. In 2021, Brazil added the imports of medical devices by 7.3%, reaching US$ 6.2 billion. The Government of Brazil is a key buyer of healthcare products, which supply them to the public healthcare system. As per the International Trade Administration ~US$ 317 million CBDL in vitro diagnostic reagents and equipment were imported into Brazil in 2021. Thus, the developing economy and growing diabetes prevalence fuels the in-vitro diagnostics market growth in Brazil.
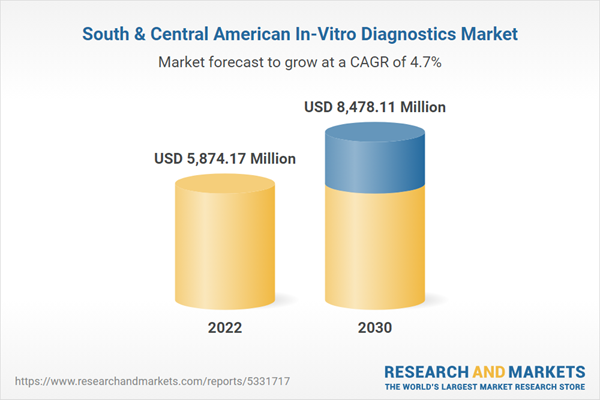
Sout
h & Central America In-Vitro Diagnostics Market Revenue and Forecast to 2030 (US$ Million)
South & Central America In-Vitro Diagnostics Market Segmentation
The South & Central America in-vitro diagnostics market is segmented into product & services, technology, application, end user, and country.Based on product & services, the South & Central America in-vitro diagnostics market is segmented into reagents & kits, instruments, and software & services. The reagents & kits segment held the largest share of the South & Central America in-vitro diagnostics market in 2022.
Based on technology, the South & Central America in-vitro diagnostics market is segmented into immunoassay/ immunochemistry, clinical chemistry, molecular diagnostics, microbiology, blood glucose self-monitoring, coagulation & hemostasis, hematology, urinalysis, and others. The immunoassay/ immunochemistry segment held the largest share of the South & Central America in-vitro diagnostics market in 2022.
Based on application, the South & Central America in-vitro diagnostics market is segmented into infectious diseases, diabetes, oncology, cardiology, autoimmune diseases, nephrology, and others. The infectious diseases segment held the largest share of the South & Central America in-vitro diagnostics market in 2022.
Based on end user, the South & Central America in-vitro diagnostics market is segmented into hospitals, laboratories, homecare, and others. The hospitals segment held the largest share of the South & Central America in-vitro diagnostics market in 2022.
Based on country, the South & Central America in-vitro diagnostics market is segmented int o Brazil, Argentina, and the Rest of South & Central America. Brazil dominated the South & Central America in-vitro diagnostics market in 2022.
Abbott Laboratories, Becton Dickinson and Co, bioMerieux SA, Bio-Rad Laboratories Inc, Danaher Corp, F. Hoffmann-La Roche Ltd, Qiagen NV, Siemens AG, Sysmex Corp, and Thermo Fisher Scientific Inc are some of the leading companies operating in the South & Central America in-vitro diagnostics market.
Reasons to Buy
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the South & Central America In-Vitro Diagnostics Market.
- Highlights key business priorities in order to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the South & Central America In-Vitro Diagnostics Market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth South & Central America market trends and outlook coupled with the factors driving the market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin security interest with respect to client products, segmentation, pricing and distribution.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd
- Danaher Corp
- Siemens AG
- Sysmex Corp
- Thermo Fisher Scientific Inc
- Becton Dickinson and Co
- bioMerieux SA
- Bio-Rad Laboratories Inc
- Qiagen NV
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 113 |
| Published | January 2024 |
| Forecast Period | 2022 - 2030 |
| Estimated Market Value ( USD | $ 5874.17 Million |
| Forecasted Market Value ( USD | $ 8478.11 Million |
| Compound Annual Growth Rate | 4.7% |
| No. of Companies Mentioned | 10 |