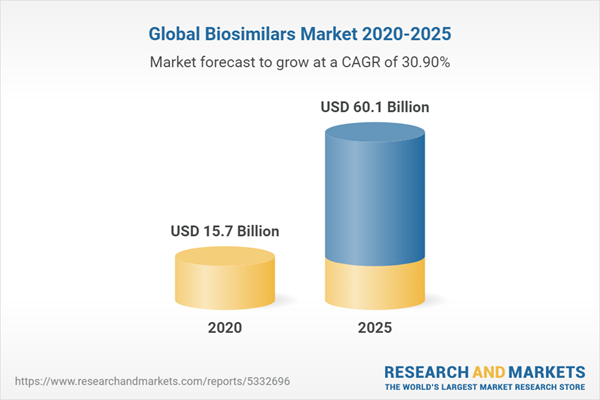
The global biosimilars market should reach $60.1 billion by 2025 from $15.7 billion in 2020 at a compound annual growth rate (CAGR) of 30.9% for the forecast period of 2020 to 2025.
Report Scope
Biosimilar drugs have gained immense popularity because of their impact on the lives of many patients. These drugs belong to several drug classes including hormones, interferons, growth factors (colony stimulating factors, erythropoietin) and monoclonal antibodies, among others. The use of these drugs has aided in the affordable treatment of many life-threatening diseases ranging from cancer and diabetes to chronic inflammatory diseases such as rheumatoid arthritis, psoriatic arthritis and others. The high cost of branded biologics has made biosimilars a lucrative alternative for affordable treatment. According to this report on biosimilars, the global market for biosimilars is expected to reach close to $20.8 billion by 2022, growing at a CAGR of 30.5%. Other drivers for this market include rising aging populations, patent expirations of many blockbuster drugs, and better healthcare provisions.
This updated report provides an in-depth analysis of the market for biosimilars in a global context, including market forecasts and sales through 2025. This study surveys the market for biosimilars in all the geographic regions including North America, Europe, the developed rest of the world (RoW), and emerging markets. The emerging markets include countries like India, China, Taiwan, Africa and Latin America.
The report provides an analysis of the market for biosimilars in various segments, for instance, by type, by region and by application/disease category. The report will include a detailed overview about the subject wherein the classification of biosimilar drugs along with their approval mechanisms, clinical trials and applications under review are elaborated upon.
The report also provides relevant patent analysis in both the U.S. and the European Union and comprehensive profiles of companies that lead the biosimilar drugs industry. The industry structure, focusing on the important biosimilar drug manufacturers/suppliers and their market shares and product offerings, is analyzed. This report also discusses the current market situation by elaborating upon the market drivers, restraints, challenges and opportunities. Separate chapters discuss the regulatory aspects and clinical trials. The latest news pieces including new products, new indications, mergers and acquisitions in the market are also dealt with in sufficient detail.
Excluded from this report are biobetters, generics of small molecule drugs and the biogenerics pertaining to vaccines and blood products. The different requirements for approval and bioequivalence between generics and biosimilars put them in an entirely different regime.
The Report Includes
- 69 data tables and 73 additional tables
- An updated review and industry insights of the global biosimilars market
- Analyses of the global market trends, with data from 2018 to 2020, and projections of compound annual growth rates (CAGRs) through 2025
- Highlights of the emerging market regulations, clinical trials, and new products launches; and their impact on the stakeholders in this market
- Discussion of the biosimilars industry structure, opportunities and complexities, regulatory updates and penetration of biosimilar product classes in various regions worldwide
- Estimation of current market size and potential growth forecast for biosimilars market, and corresponding market share analysis by product type, application/disease category and geographic region
- A detailed review of patents issued for biosimilars by different assignee categories
- Impact analysis of COVID-19 pandemic on the growth of biosimilars market as compared to overall pharmaceuticals industry with respect to clinical trials/approvals
- Company profiles of the major market players, including Amgen Inc., Boehringer Ingelheim, Cipla Ltd., Dr Reddy’s Laboratories Ltd., Lupin Ltd., Pfizer Inc. and Zydus Cadila
Table of Contents
Chapter 1 Introduction
- Study Goals and Objectives
- Reasons for Doing This Study
- Scope of Report
- Information Sources
- Methodology
- Geographic Breakdown
- Analyst's Credentials
- Custom Research
- Related Reports
Chapter 2 Summary and Highlights
Chapter 3 Market and Technology Background
- Terminology of Biosimilars
- Definitions of Biosimilars
- Development of a Biosimilar
- Clone Development and Selection
- Manufacture of a Biosimilar
- Preclinical Studies and Validation of a Biosimilar
- Clinical Trials
- Approval by Regulatory Agencies
- Pharmacovigilance/Post-Approval Monitoring
- Biosimilars Versus Biobetters
- Popular Types of Biosimilars
- Recombinant Hormones
- Recombinant Growth Factors
- Monoclonal Antibodies (mAbs)
- Fusion Proteins
- Interferons
- Low Molecular Weight Heparins (LMWHs)
Chapter 4 Market Breakdown by Type of Biosimilar
- Global Market for Biosimilars by Type
- Market Shares
- Recombinant Hormone Biosimilars
- Recombinant Growth Factor Biosimilars
- Monoclonal Antibody Biosimilars
- Fusion Protein Biosimilars
- Interferons
- LMWHs
Chapter 5 Market Breakdown by Therapeutic Application
- Global Market for Therapeutic Applications of Biosimilars
- Market Revenue
- Cancer and Related Disorders
- Diabetes
- Anemia
- Growth Hormone Deficiency
- Autoimmune Diseases
- Infectious Diseases
- Other Types of Diseases
Chapter 6 Market Breakdown by Region
- Introduction
- Global Trends
- Factors Impacting the Global Market for Biosimilars
- Market by Geographical Region
- North America
- Europe
- Developed Countries in the RoW Region
- Emerging Markets
- Recombinant Hormone Biosimilars
- Market Revenue
- Market Shares
- Somatotropin Biosimilars
- Follitropin Alfa Biosimilars
- Insulin Biosimilars
- Teriparatide Biosimilars
- Recombinant Growth Factor Biosimilars
- Market Revenue
- Market Shares
- Erythropoietin Biosimilars
- Darbepoetin Alfa Biosimilars
- Filgrastim Biosimilars
- Pegfilgrastim Biosimilars
- Monoclonal Antibody Biosimilars
- Market Revenue
- Market Shares
- Adalimumab Biosimilars
- Bevacizumab Biosimilars
- Infliximab Biosimilars
- Ranibizumab Biosimilars
- Rituximab Biosimilars
- Trastuzumab Biosimilars
- Fusion Protein Biosimilars
- Market Overview
- Market Revenue
- Market Shares
- Interferon Biosimilars
- Market Overview
- Market Revenue
- Market Shares
- Low Molecular Weight Heparin (Enoxaparin Sodium) Biosimilars
- Market Overview
- Market Revenue
- Market Shares
Chapter 7 Industry Structure
- Types of Market Players
- Established Biologics Companies
- Established Generics Companies
- Bio-intellectual Companies
- Opportunistic Companies
- Prioritizers
- Emerging Trends in the Biosimilar Industry
- Collaborations and Partnerships
- Mergers and Acquisitions
- Manufacturing Facilities of Major Market Players
- Contract Manufacturing Organizations (CMOs)
- Leading Manufacturers/Suppliers of Biosimilar Drugs
- Recombinant Hormones
- Recombinant Growth Factors
- Monoclonal Antibodies
- Fusion Proteins
- Interferons
- LMWHs
Chapter 8 Regulatory Aspects
- Biosimilar Regulations in Europe
- Impact of Brexit
- Biosimilar Regulations in the U.S.
- Principle of Exclusivity
- Requirement of 351(k) Application
- Approaches to Demonstrate Biosimilarity
- Biosimilar Regulations in Emerging Markets
- Biosimilar Guidelines in India
- Biosimilar Regulations in China
- Biosimilar Regulations in South Korea
- Biosimilar Regulations in Japan
- Biosimilar Regulations in Australia
- Naming of Biosimilars
- FDA Naming Convention
- WHO Naming Convention
- EMA Naming Convention
- Interchangeability and Substitution
Chapter 9 Patent Analysis
- Patent Regulations in Favor of Branded Biologics
- Market Exclusivity
- Patent Regulations in Favor of Biosimilars
- Market Exclusivity
- Product Versus Process Patents
- Patent Activity in Biosimilars, 2018 Through February 2021
- U.S. Patents
- International Patents
Chapter 10 Clinical Trials
- Biosimilars in Clinical Trials
- Clinical Trials Analysis
- Clinical Trials by Recruitment Status
- Clinical Trials by Type of Study
- Clinical Trials by Study Phase
- Clinical Trials by Biosimilar Active Substance
- Clinical Trials by Therapeutic Area
- Biosimilar Candidates in Ongoing Clinical Trials
Chapter 11 Analysis of Market Forces
- Market Dynamics
- Market Drivers
- Market Restraints
- Opportunities
- Issues
Chapter 12 Company Profiles
- 3S Bio Inc.
- Alvotech
- Amgen Inc.
- Biocad
- Biocon Ltd.
- Bioeq Gmbh
- Biopartners Gmbh
- Biosidus S.A.
- Bio-Thera Solutions Ltd.
- Bioxpress Therapeutics S.A.
- Blau Farmaceutica S/A
- Boehringer Ingelheim Pharmaceuticals Inc.
- Chong Kun Dang Pharmaceutical Corp.
- Celltrion Inc.
- Cipla Ltd.
- Cinnagen
- Coherus Biosciences Inc.
- DM Bio Ltd.
- Dong-A Socio Holdings
- Dr. Reddy's Laboratories Ltd.
- Emcure Pharmaceuticals Ltd.
- Fresenius Kabi Ag
- Formycon Ag
- Gedeon Richter Plc
- Genescience Pharmaceuticals Co., Ltd.
- GC Pharma (Formerly Gress Cross Corp.)
- Hangzhou Jiuyuan Gene Engineering Co., Ltd.
- Hanwha Chemical Corp.
- Harvest Moon Pharmaceuticals U.S.A. Inc.
- Innovent Biologics Co., Ltd.
- Intas Pharmaceuticals Ltd.
- JHL Biotech Inc.
- JCR Pharmaceuticals Co., Ltd.
- Kissei Pharmaceutical Co., Ltd.
- LG Chem (Formerly Lg Life Sciences)
- Lupin Ltd.
- Mabxience S.A.
- Mylan N.V. (Now Viatris Inc.)
- Nanogen Biopharmaceutical Co.
- Neuclone
- Nippon Kayaku Co., Ltd.
- Pfenex Inc.
- Pfizer Inc.
- Pharmapark Llc
- Prestige Biopharma Pte Ltd.
- Probiomed S.A.
- Reliance Life Sciences
- Samsung Bioepis Co., Ltd.
- Sandoz International Gmbh
- Shanghai Henlius Biotech Inc.
- Stada Arzneimittel Ag
- Tanvex Biopharma Inc.
- Teva Pharmaceuticals Industries Ltd.
- USV Pvt. Ltd.
- Wockhardt Ltd.
- Xiamen Amoytop Biotech Co., Ltd.
- Zydus Cadila
Chapter 13 Appendix: Acronyms
List of Tables
Summary Table: Global Market for Biosimilars, by Type, Through 2025
Table 1: Differences between Generic and Biosimilar Drugs
Table 2: EMA Terminology Related to Biosimilars
Table 3: FDA Terminology Related to Biosimilars
Table 4: WHO Terminology Related to Biosimilars
Table 5: Differences between <i>E. coli<i/> and CHO Cell Lines
Table 6: List of Biosimilars Produced in E. coli
Table 7: Key Differences between Approval Pathways for Biosimilars in the EU and the U.S.
Table 8: Biosimilars Versus Biobetters
Table 9: Examples of Approved Biobetters
Table 10: Representative Biobetters in Clinical Trials
Table 11: Biosimilar Recombinant Hormones
Table 12: Biosimilar Recombinant Growth Factors
Table 13: Biosimilar Monoclonal Antibodies
Table 14: Biosimilar Fusion Proteins
Table 15: Biosimilar Interferons
Table 16: Biosimilar LMWHs
Table 17: Number of Approved Biosimilars, by Reference Product, February 2021
Table 18: Global Market for Biosimilars, by Type, Through 2025
Table 19: Global Market Share of Biosimilars, by Type, 2019
Table 20: Global Market for Recombinant Hormone Biosimilars, by Type, Through 2025
Table 21: Pegfilgrastim Biosimilars Approved, 2018-2020
Table 22: Global Market for Recombinant Growth Factor Biosimilars, by Type, Through 2025
Table 23: Branded Monoclonal Antibody Drugs Used as Reference Drugs for Biosimilars
Table 24: FDA-approved Adalimumab Biosimilars
Table 25: Trastuzumab Biosimilars
Table 26: Global Market for Monoclonal Antibody Biosimilars, by Type, Through 2025
Table 27: Global Market for Fusion Protein Biosimilars, by Type, Through 2025
Table 28: Global Market for Interferon Biosimilars, by Type, Through 2025
Table 29: Global Market for LMWH Biosimilars, Through 2025
Table 30: Approved Biosimilars, by Therapeutic Application, March 2021
Table 31: Global Market for Biosimilars, by Therapeutic Application, Through 2025
Table 32: Global Market Share of Biosimilars, by Therapeutic Application, 2019
Table 33: Global Market for Biosimilars in the Treatment of Cancer, by Region, Through 2025
Table 34: Global Market for Biosimilars in the Treatment of Diabetes, by Region, Through 2025
Table 35: Global Market for Biosimilars in the Treatment of Anemia, by Region, Through 2025
Table 36: Global Market for Biosimilars in the Treatment of Growth Hormone Deficiency, by Region, Through 2025
Table 37: Global Market for Biosimilars in the Treatment of Autoimmune Diseases, by Region, Through 2025
Table 38: Global Market for Biosimilars in the Treatment of Infectious Diseases, by Region, Through 2025
Table 39: Global Market for Biosimilars in the Treatment of Other Types of Diseases, by Region, Through 2025
Table 40: Biosimilar Drug Approvals, by Country, 2005-2020
Table 41: Global Market for Biosimilars, by Region, Through 2025
Table 42: Global Market Share of Biosimilars, by Region, 2019
Table 43: Global Market for Recombinant Hormone Biosimilars, by Region, Through 2025
Table 44: Global Market Share of Recombinant Hormone Biosimilars, by Region, 2019
Table 45: Global Market for Somatotropin Biosimilars, by Region, Through 2025
Table 46: Global Market for Follitropin Alfa Biosimilars, by Region, Through 2025
Table 47: Global Market for Insulin Biosimilars, by Region, Through 2025
Table 48: Global Market for Teriparatide Biosimilars, by Region, Through 2025
Table 49: Global Market for Recombinant Growth Factor Biosimilars, by Region, Through 2025
Table 50: Global Market Share of Recombinant Growth Factor Biosimilars, by Region, 2019
Table 51: Global Market for Erythropoietin Biosimilars, by Region, Through 2025
Table 52: Global Market for Darbepoetin Alfa Biosimilars, by Region, Through 2025
Table 53: Global Market for Filgrastim Biosimilars, by Region, Through 2025
Table 54: Pegfilgrastim Pipeline
Table 55: Global Market for Pegfilgrastim Biosimilars, by Region, Through 2025
Table 56: Global Market for Monoclonal Antibody Biosimilars, by Region, Through 2025
Table 57: Global Market Share of Monoclonal Antibody Biosimilars, by Region, 2019
Table 58: Global Market for Adalimumab Biosimilars, by Region, Through 2025
Table 59: Global Market for Bevacizumab Biosimilars, by Region, Through 2025
Table 60: Global Market for Infliximab Biosimilars, by Region, Through 2025
Table 61: Ranibizumab Biosimilars Pipeline
Table 62: Global Market for Ranibizumab Biosimilars, by Region, Through 2025
Table 63: Global Market for Rituximab Biosimilars, by Region, Through 2025
Table 64: Global Market for Trastuzumab Biosimilars, by Region, Through 2025
Table 65: Global Market for Fusion Protein Biosimilars, by Region, Through 2025
Table 66: Global Market Share of Fusion Protein Biosimilars, by Region, 2019
Table 67: Global Market for Interferon Biosimilars, by Region, Through 2025
Table 68: Global Market Share of Interferon Biosimilars, by Region, 2019
Table 69: Global Market for Low Molecular Weight Heparin (Enoxaparin Sodium) Biosimilars, by Region, Through 2025
Table 70: Global Market Share of Low Molecular Weight Heparin (Enoxaparin Sodium) Biosimilars, by Region, 2019
Table 71: Collaborations and Partnerships in the Market for Biosimilars, 2018-March 2021
Table 72: Mergers and Acquisitions in the Market for Biosimilars, 2018-March 2021
Table 73: Manufacturing Facilities of Major Companies in Biosimilars
Table 74: Leading Manufacturers/Suppliers of Recombinant Hormone Biosimilars, 2019
Table 75: Market Share of Leading Manufacturers/Suppliers of Recombinant Hormone Biosimilars, 2019
Table 76: Leading Manufacturers/Suppliers of Recombinant Growth Factor Biosimilars, 2019
Table 77: Market Share of Leading Manufacturers/Suppliers of Recombinant Growth Factor Biosimilars, 2019
Table 78: Leading Manufacturers/Suppliers of Monoclonal Antibody Biosimilars, 2019
Table 79: Market Share of Leading Manufacturers/Suppliers of Monoclonal Antibody Biosimilars, 2019
Table 80: Leading Manufacturers/Suppliers of Fusion Protein Biosimilars, 2019
Table 81: Market Share of Leading Manufacturers/Suppliers of Fusion Protein Biosimilars, 2019
Table 82: Leading Manufacturers/Suppliers of interferons Biosimilars, 2019
Table 83: Market Share of Leading Manufacturers/Suppliers of Interferons Biosimilars, 2019
Table 84: Leading Manufacturers/Suppliers of LMWH Biosimilars, 2019
Table 85: Market Share of Leading Manufacturers/Suppliers of LMWH Biosimilars, 2019
Table 86: Product-specific Biosimilar Guidelines, EMA
Table 87: Related Biosimilar Guidelines, EMA
Table 88: Refused/Withdrawn Biosimilar Drugs, EMA
Table 89: FDA Guidance on Biosimilars, February 2021
Table 90: Biosimilar Products Approved in China
Table 91: Biosimilar Products Approved in Korea, 2018-February 2021
Table 92: Biosimilar Products Approved in Japan, 2018-February 2021
Table 93: Biosimilar Products Approved in Australia, 2018-February 2021
Table 94: FDA Recommendations for a Proposed Suffix in a Biosimilar Product Name
Table 95: Approved Biosimilars with Proper and Proprietary Names
Table 96: U.S. Patent Expirations and Biosimilars
Table 97: Pending Biosimilar Patent Litigation
Table 98: Inter Partes Review (IPR) Challenges by Biosimilar Developers, 2018-February 2021
Table 99: Patent Litigation in Favor of Biosimilar Manufacturers
Table 100: Patents Issued on Biosimilars, by Year, 2018-February 2021
Table 101: Patents Issued on Biosimilars, by Type, 2018-February 2021
Table 102: Patents Issued on Biosimilars, by Company, 2018-February 2021
Table 103: Patents Issued on Biosimilars, by Assignee’s Country, 2018-February 2021
Table 104: Patents Issued on Biosimilars, by Type of Assignee, 2018-February 2021
Table 105: WIPO Patents Issued on Biosimilars, by Year, 2018-February 2021
Table 106: WIPO Patents Issued on Biosimilars, by Type, 2018-February 2021
Table 107: WIPO Patents Issued on Biosimilars, by Company, 2018-February 2021
Table 108: WIPO Patents Issued on Biosimilars, by Country/Patent Office Where Filed, 2018-February 2021
Table 109: Major Biosimilar Candidates with Patent Expirations
Table 110: Clinical Trials on Biosimilars, by Recruitment Status, 2018-February 2020
Table 111: Clinical Trials on Biosimilars, by Type of Study
Table 112: Clinical Trials on Biosimilars, by Study Phase
Table 113: Clinical Trials on Biosimilars, by Biosimilar Active Substance
Table 114: Clinical Trials on Biosimilars, by Therapeutic Area
Table 115: Ongoing Clinical Trials on Biosimilar Candidates
Table 116: Biosimilars in Clinical Trials, by Sponsor
Table 117: Discounts and Prices of Biosimilar Drugs at Launch
Table 118: 3S Bio’s Biosimilar Pipeline
Table 119: Alvotech’s Biosimilar Partnerships
Table 120: Amgen’s Biosimilar Pipeline
Table 121: Biocad’s Biosimilar Pipeline
Table 122: Biocon’s Biosimilars
Table 123: Bioeq’s Biosimilar Pipeline
Table 124: Bio-Thera Solutions’ Biosimilar Pipeline
Table 125: BioXpress Therapeutics’ Biosimilar Development Programs
Table 126: CKD Pharmaceuticals’ Biosimilar Development Programs
Table 127: Celltrion’s Biosimilar Development Programs
Table 128: Coherus Biosciences’ Biosimilar Development Programs
Table 129: DM Bio’s Biosimilar Development Programs
Table 130: Dong A-ST’s Biosimilar Development Programs
Table 131: Dr Reddy’s Laboratories’ Biosimilar Development Programs
Table 132: Fresenius Kabi’s Biosimilar Development Programs
Table 133: Formycon’s Biosimilar Development Programs
Table 134: Gedeon Richter’s Biosimilar Development Programs
Table 135: Lupin’s Biosimilar Development Programs
Table 136: Mylan’s Biosimilar Development Programs
Table 137: Samsung Bioepis’ Biosimilar Development Programs
Table 138: Henlius Biotech’s Biosimilar Development Programs
Table 139: Tanvex Biopharma’s Biosimilar Development Programs
Table 140: Teva Pharmaceuticals’ Biosimilar Development Programs
Table 141: Acronyms Used in the Market for Biosimilars
List of Figures
Summary Figure: Global Market for Biosimilars, by Type, 2018-2025
Figure 1: Depiction of a Typical Biosimilar Drug Development Timeline
Figure 2: Global Market for Biosimilars, by Type, 2018-2025
Figure 3: Global Market Share of Biosimilars, by Type, 2019
Figure 4: Global Market for Recombinant Hormone Biosimilars, by Type, 2018-2025
Figure 5: Global Market for Recombinant Growth Factor Biosimilars, by Type, 2018-2025
Figure 6: Global Market for Monoclonal Antibody Biosimilars, by Type, 2018-2025
Figure 7: Global Market for Fusion Protein Biosimilars, by Type, 2018-2025
Figure 8: Global Market for Interferon Biosimilars, by Type, 2018-2025
Figure 9: Global Market for LMWH Biosimilars, by Type, 2018-2025
Figure 10: Global Market for Biosimilars, by Therapeutic Application, 2018-2025
Figure 11: Global Market Share of Biosimilars, by Therapeutic Application, 2019
Figure 12: Global Market for Biosimilars in the Treatment of Cancer, by Region, 2018-2025
Figure 13: Global Market for Biosimilars in the Treatment of Diabetes, by Region, 2018-2025
Figure 14: Global Market for Biosimilars in the Treatment of Anemia, by Region, 2018-2025
Figure 15: Global Market for Biosimilars in the Treatment of Growth Hormone Deficiency, by Region, 2018-2025
Figure 16: Global Market for Biosimilars in the Treatment of Autoimmune Diseases, by Region, 2018-2025
Figure 17: Global Market for Biosimilars in the Treatment of Infectious Diseases, by Region, 2018-2025
Figure 18: Global Market for Biosimilars in the Treatment of Other Types of Diseases, by Region, 2018-2025
Figure 19: Global Market for Biosimilars, by Region, 2018-2025
Figure 20: Global Market Share of Biosimilars, by Region, 2019
Figure 21: Global Market for Recombinant Hormone Biosimilars, by Region, 2018-2025
Figure 22: Global Market Share of Recombinant Hormone Biosimilars, by Region, 2019
Figure 23: Global Market for Somatotropin Biosimilars, by Region, 2018-2025
Figure 24: Global Market for Follitropin Alfa Biosimilars, by Region, 2018-2025
Figure 25: Global Market for Insulin Biosimilars, by Region, 2018-2025
Figure 26: Global Market for Teriparatide Biosimilars, by Region, 2018-2025
Figure 27: Global Market for Recombinant Growth Factor Biosimilars, by Region, 2018-2025
Figure 28: Global Market Share of Recombinant Growth Factor Biosimilars, by Region, 2019
Figure 29: Global Market for Erythropoietin Biosimilars, by Region, 2018-2025
Figure 30: Global Market for Darbepoetin Alfa Biosimilars, by Region, 2018-2025
Figure 31: Global Market for Filgrastim Biosimilars, by Region, 2018-2025
Figure 32: Global Market for Pegfilgrastim Biosimilars, by Region, 2018-2025
Figure 33: Global Market for Monoclonal Antibody Biosimilars, by Region, 2018-2025
Figure 34: Global Market Share of Monoclonal Antibody Biosimilars, by Region, 2019
Figure 35: Global Market for Adalimumab Biosimilars, by Region, 2018-2025
Figure 36: Global Market for Bevacizumab Biosimilars, by Region, 2018-2025
Figure 37: Global Market for Infliximab Biosimilars, by Region, 2018-2025
Figure 38: Global Market for Ranibizumab Biosimilars, by Region, 2018-2025
Figure 39: Global Market for Rituximab Biosimilars, by Region, 2018-2025
Figure 40: Global Market for Trastuzumab Biosimilars, by Region, 2018-2025
Figure 41: Global Market for Fusion Protein Biosimilars, by Region, 2018-2025
Figure 42: Global Market Share of Fusion Protein Biosimilars, by Region, 2019
Figure 43: Global Market for Interferon Biosimilars, by Region, 2018-2025
Figure 44: Global Market Share of Interferon Biosimilars, by Region, 2019
Figure 45: Global Market for Low Molecular Weight Heparin (Enoxaparin Sodium) Biosimilars, by Region, 2018-2025
Figure 46: Global Market Share of Low Molecular Weight Heparin (Enoxaparin Sodium) Biosimilars, by Region, 2019
Figure 47: Market Share of Leading Manufacturers/Suppliers of Recombinant Hormone Biosimilars, 2019
Figure 48: Market Share of Leading Manufacturers/Suppliers of Recombinant Growth Factor Biosimilars, 2019
Figure 49: Market Share of Leading Manufacturers/Suppliers of Monoclonal Antibody Biosimilars, 2019
Figure 50: Market Share of Leading Manufacturers/Suppliers of Fusion Protein Biosimilars, 2019
Figure 51: Market Share of Leading Manufacturers/Suppliers of Interferon Biosimilars, 2019
Figure 52: Market Share of Leading Manufacturers/Suppliers of LMWH Biosimilars, 2019
Figure 53: Patents Issued on Biosimilars, by Year, 2018-February 2021Figure 54: Patents Issued on Biosimilars, by Assignee’s Country, 2018-February 2021
Figure 55: Patents Issued on Biosimilars, by Type of Assignee, 2018-February 2021
Figure 56: WIPO Patents Share Issued on Biosimilars, by Year, 2018-February 2021
Figure 57: WIPO Patents Issued on Biosimilars, by Type, 2018-February 2021
Figure 58: WIPO Patents Issued on Biosimilars, by Country/Patent Office Where Filed, 2018-February 2021
Figure 59: Clinical Trials on Biosimilars, by Recruitment Status, 2018-February 2020
Figure 60: Clinical Trials on Biosimilars, by Type of Study
Figure 61: Clinical Trials on Biosimilars, by Study Phase
Figure 62: Clinical Trials on Biosimilars, by Active Substance
Figure 63: Clinical Trials on Biosimilars, by Therapeutic Area
Figure 64: SWOT Analysis of the Market for Biosimilars
Figure 65: Biosimilar Discounts at Product Launch"
Companies Mentioned
- 3S Bio Inc.
- Alvotech
- Amgen Inc.
- Bio-Thera Solutions Ltd.
- Biocad
- Biocon Ltd.
- Bioeq Gmbh
- Biopartners Gmbh
- Biosidus S.A.
- Bioxpress Therapeutics S.A.
- Blau Farmaceutica S/A
- Boehringer Ingelheim Pharmaceuticals Inc.
- Celltrion Inc.
- Chong Kun Dang Pharmaceutical Corp.
- Cinnagen
- Cipla Ltd.
- Coherus Biosciences Inc.
- DM Bio Ltd.
- Dong-A Socio Holdings
- Dr. Reddy’S Laboratories Ltd.
- Emcure Pharmaceuticals Ltd.
- Formycon Ag
- Fresenius Kabi Ag
- Gc Pharma (Formerly Gress Cross Corp.)
- Gedeon Richter Plc
- Genescience Pharmaceuticals Co., Ltd.
- Hangzhou Jiuyuan Gene Engineering Co., Ltd.
- Hanwha Chemical Corp.
- Harvest Moon Pharmaceuticals U.S.A. Inc.
- Innovent Biologics Co., Ltd.
- Intas Pharmaceuticals Ltd.
- JCR Pharmaceuticals Co., Ltd.
- JHL Biotech Inc.
- Kissei Pharmaceutical Co., Ltd.
- LG Chem (Formerly Lg Life Sciences)
- Lupin Ltd.
- Mabxience S.A.
- Mylan N.V. (Now Viatris Inc.)
- Nanogen Biopharmaceutical Co.
- Neuclone
- Nippon Kayaku Co., Ltd.
- Pfenex Inc.
- Pfizer Inc.
- Pharmapark Llc
- Prestige Biopharma Pte Ltd.
- Probiomed S.A.
- Reliance Life Sciences
- Samsung Bioepis Co., Ltd.
- Sandoz International Gmbh
- Shanghai Henlius Biotech Inc.
- Stada Arzneimittel Ag
- Tanvex Biopharma Inc.
- Teva Pharmaceuticals Industries Ltd.
- USV Pvt. Ltd.
- Wockhardt Ltd.
- Xiamen Amoytop Biotech Co., Ltd.
- Zydus Cadila
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 358 |
| Published | May 2021 |
| Forecast Period | 2020 - 2025 |
| Estimated Market Value ( USD | $ 15.7 Billion |
| Forecasted Market Value ( USD | $ 60.1 Billion |
| Compound Annual Growth Rate | 30.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 57 |