Chapter 2 Summary and Highlights
- Market Outlook
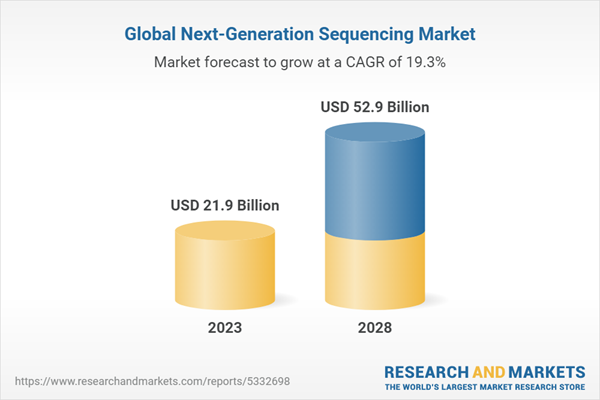
- Market Summary
Chapter 4 Technology Background
- Importance of DNA
- Genetic Variation and Analysis
- Genetic Analysis Technologies
- Sequencing in Clinical Applications
- Sequencing Technologies
- History of DNA Sequencing
- Sanger Sequencing Technology
- NGS Platforms
- Short-Read Platforms
- Long-Read Platforms
- Informatics Technologies
- Base Calling
- Mapping to a Reference Sequence
- Variant Analysis
- Artificial Intelligence Technologies
- Clinical Sequencing Technology Challenges
Chapter 5 Clinical NGS Initiatives and Emerging Technologies
- R&D Initiatives and Programs
- 1+ Million Genomes
- Access to Treatment and Testing (ACTT)
- Access to Comprehensive Genomic Profiling Coalition (ACGP)
- Africa Pathogen Genomics Initiative
- Blood Profiling Atlas
- Cancer-ID
- Cancer Moon Shots Program
- China Precision Medicine Initiative
- ClinGen
- CTC Trap Consortium
- diaRNAgnosis Project
- Early Cancer Detection Consortium
- EpiFemCare
- France Genomic Medicine Plan
- Friends of Cancer Research Project
- Genomic Medicine Sweden
- HCA-Organoid
- Human Cell Atlas
- Human Immunomics Initiative
- Immunomonitor Consortium
- Integration of Imaging and Fluid-Based Tumor Monitoring in Cancer Therapy Program
- Intervene
- Liquid Biopsies and Imaging for Improved Cancer Care
- Liquid Biopsy-Based Malignant Tumor Early Screening Technology Research and Development Project
- Liver Cancer Early Screening Comprehensive Prevention and Control Project
- Lung Cancer Genomic Screening Project for Individualized Medicine in Asia
- Million Veteran Program
- Medical Genome Initiative
- MedSeq
- Precision Medicine Initiative
- Prompt
- QuIP Project
- SPHERES
- Target ALS Diagnosis Initiative
- TopMed
- Treehouse Childhood Cancer Initiative
- Very Rare Cancer Consortium
- Worldwide Innovative Networking (WIN) Consortium
- Single-Cell Research
- Cambridge Single-Cell Analysis Core Facility
- Harvard Medical School Single-Cell Core
- Mayo Medical Genome Facility
- National Center for Single-Cell Biology
- Next-Generation Single-Cell Analysis Program
- Single-Cell Analysis Core
- UC San Francisco Single-Cell Analysis Center
- The Wistar Institute of Anatomy and Biology
- Population Sequencing Projects
Chapter 6 Clinical NGS Applications
- Introduction
- Cancer Applications
- Precision Medicine
- Liquid Biopsy Biomarkers
- Mendelian Disorders Applications
- Reproductive Health Applications
- Noninvasive Prenatal Testing
- Newborn Screening
- Microbiology and Infectious Disease Applications
- Complex Disorders Applications
Chapter 7 Clinical NGS Industry
- Sequencing Instrument Industry
- Companies
- Third-Generation Sequencing Industry
- Sequencing Informatics Industry
- Target Enrichment and Amplification Industry
- CTC Capture and Detection Industry
- Liquid Biopsy Assay Industry
- Liquid Biopsy Cancer Screening/Early Detection Industry
- Health-Focused DTC Genetic Testing Industry: Two Market Models
- DTC Clinical Health Genetic Testing Industry
- Noninvasive Prenatal Testing (NIPT) Industry
Chapter 8 ESG Development
- Introduction to ESG
- The Sustainability of NGS in Industry
- Case Study
- Publisher Viewpoint
Chapter 9 Acquisitions and Strategic Alliances
- Acquisitions
- Strategic Alliances
- Key Trends
Chapter 10 Clinical NGS Markets
- Market Dynamics
- Market Drivers
- Impact of COVID-19 on the Clinical NGS Markets
- Clinical NGS Market by Disease Category
- Clinical NGS Market by Test Complexity
- Clinical NGS Market by Purpose of Test
- Clinical NGS Market in Oncology
- Market by Indication in Oncology
- NGS Market in Oncology by Purpose of Test
- NGS Market in Oncology by Test Complexity
- Clinical NGS Market in Cardiology
- NGS Market in Cardiology by Purpose of Test
- NGS Market in Cardiology by Test Complexity
- Clinical NGS Market in Mendelian Disorders
- NGS Market in Mendelian Disorders by Test Complexity
- Clinical NGS Market in Metabolic/Immune Disorders
- NGS Market in Metabolic/Immune Disorders by Purpose of Test
- NGS Market in Metabolic/Immune Disorders by Test Complexity
- Clinical NGS Market in Neurology
- NGS Market in Neurology by Test Purpose
- NGS Market in Neurology by Test Complexity
- Clinical NGS Market in Reproductive Health
- Reproductive Health Market by Test Application
- NGS Market in Reproductive Health by Purpose of Test
- NGS Market in Reproductive Health by Test Complexity
- Clinical NGS Market in Microbiology and Infectious Diseases
- NGS Market in Microbiology and Infectious Diseases by Purpose of Test
- NGS Market in Microbiology and Infectious Diseases by Test Complexity
- Clinical NGS Market in Transplantation
- NGS Market in Transplantation by Organ Type
- NGS Market in Transplantation by Purpose of Test
- NGS Market in Transplantation by Test Complexity
- Clinical NGS Market by Region
- Global Market for NGS in Oncology by Region
- Global Market for NGS in Reproductive Health by Region
- Global Market for NGS in Transplantation by Region
Chapter 12 Company Profiles
List of Tables
Table A: Scope of the Report
Summary Table: Global Market for Clinical Applications of Next-Generation Sequencing, by Disease Class, Through 2028
Table 1: Global Market for Clinical Next-Generation Sequencing, by Disease Type, Through 2028
Table 2: Forces Influencing Growth in the Clinical NGS Market
Table 3: Key Trends in the Clinical NGS Testing Market
Table 4: Clinical NGS Industry Subsectors
Table 5: DNA Chemical Building Blocks
Table 6: DNA Translation and Transcription
Table 7: Long-Range DNA Structure
Table 8: Genetic Variant Types
Table 9: Molecular Diagnostic Technology Platforms
Table 10: Genome Coverage of PCR, Microarray and NGS Technology Platforms
Table 11: Genetic Testing Coverage
Table 12: Key Near- to Mid-term Segments of NGS Diagnostics
Table 13: DNA Sequencing Historical Timeline, 1977-2023
Table 14: Sequencing Technology Development, 1990-2020
Table 15: Cost of Sequencing a Single Human Genome, 2001-2022
Table 16: Summary of Sanger Sequencing
Table 17: Improvements in Sanger Sequencing
Table 18: NGS Technologies
Table 19: Illumina NGS Workflow
Table 20: Thermo Fisher Scientific NGS Workflow
Table 21: NGS Bioinformatics Workflow
Table 22: Sequence Annotation Steps
Table 23: Types of AI in Healthcare
Table 24: Future Challenges in Implementing Clinical NGS
Table 25: Clinical NGS Research and Development Initiatives and Programs
Table 26: Single-Cell Core Research Facilities
Table 27: Population Sequencing Projects
Table 28: Clinical NGS Near- to Mid-term Applications
Table 29: Personalized Medicine Driven by Common Cancer Genetic Mutations
Table 30: Liquid Biopsy Biomarkers
Table 31: NGS-Based Cancer Diagnostics Market Segments
Table 32: Mendelian Disorders Applications
Table 33: Reproductive Health Screening Applications
Table 34: Ethical Issues Associated with NIPT
Table 35: Microbiology/Infectious Disease Applications
Table 36: Complex Disorders Applications
Table 37: Key Segments and Trends in the Clinical NGS Industry
Table 38: NGS and 3GS Industry Company Positioning
Table 39: Long-Read Sequencing Industry
Table 40: Sequencing Informatics Industry
Table 41: NGS Target Enrichment Industry
Table 42: CTC Separations Industry
Table 43: Liquid Biopsy Assay Industry: Company Focus
Table 44: Examples of NGS-Based Liquid Biopsy Market Differentiation
Table 45: Multi-Cancer Screening Test Desired Features
Table 46: Shed Rates of Various Cancers
Table 47: Cancer Screening Liquid Biopsy Industry
Table 48: Thrive Earlier Detection (Exact Sciences) Cancer Targets
Table 49: Colorectal Cancer Early Detection/Screening Liquid Biopsy Industry
Table 50: Key Clinical Trials on Single- and Multi-Cancer Liquid Biopsy Assays
Table 51: Clinical-Grade Health DTC Genetic Testing Companies
Table 52: NIPT Industry
Table 53: Key Focus Areas in ESG Metrics
Table 54: ESG Rankings for Major Companies in the NGS Arena
Table 55: Illumina 2030 ESG Targets
Table 56: Clinical NGS Industry Acquisitions, January 2019-October 2023
Table 57: Strategic Alliances in Clinical NGS, January 2019-April 2021
Table 58: Key Forces Driving Growth in Clinical NGS and Their Implications
Table 59: Global Market for Clinical NGS, by Disease Category, Through 2028
Table 60: Global Market for Clinical NGS, by Test Complexity, Through 2028
Table 61: Global Market for Clinical NGS, by Purpose of Test, Through 2028
Table 62: Limitations of Tissue Biopsy in Cancer Applications
Table 63: Low-Frequency Mutation Detection
Table 64: Global Market for Clinical NGS in Oncology, by Indication, Through 2028
Table 65: Five-Year Survival Rate for Ovarian Cancer
Table 66: National Institutes of Health Liquid Biopsy Early Detection Initiative
Table 67: Clinical Trials on Lung Cancer Liquid Biopsies
Table 68: Global Market for Clinical NGS in Oncology, by Purpose of Test, Through 2028
Table 69: MRI and Liquid Biopsy Methods
Table 70: Early Detection Tissue of Origin Approaches
Table 71: Global Market for Clinical NGS in Oncology, by Test Complexity, Through 2028
Table 72: European Society of Medical Oncology NGS Recommendations for Metastatic Cancers, August 2020
Table 73: Global Market for Clinical NGS in Cardiology Diagnostics, by Purpose of Test, Through 2028
Table 74: NGS-Based Dilated Cardiomyopathy Test Providers
Table 75: Global Market for Clinical NGS in Cardiology Diagnostics, by Test Complexity, Through 2028
Table 76: Global Market for Clinical NGS in Mendelian Disorder Diagnostics, by Purpose of Test, Through 2028
Table 77: Global Market for Clinical NGS in Mendelian Disorder Diagnostics, by Test Complexity, Through 2028
Table 78: Global Market for Clinical NGS in Metabolic and Immune Disorder Diagnostics, by Purpose of Test, Through 2028
Table 79: Multiple Sclerosis Diagnostic Technologies
Table 80: Global Market for Clinical NGS in Metabolic and Immune Disorder Diagnostics, by Test Complexity, Through 2028
Table 81: Global Market for Clinical NGS in Neurological Diagnostics, by Purpose of Test, Through 2028
Table 82: Global Market for Clinical NGS in Neurological Diagnostics, by Test Complexity, Through 2028
Table 83: Global Market for Clinical NGS in Reproductive Health, by Application, Through 2028
Table 84: Global NGS-Based NIPT Market, by Risk Type, Through 2028
Table 85: Global Market for Clinical NGS in Reproductive Health, by Purpose of Test, Through 2028
Table 86: Global Market for Clinical NGS in Reproductive Health, by Test Complexity, Through 2028
Table 87: NGS-Based Infectious Disease Landscape
Table 88: Global Market for Clinical NGS-Based Microbiology and Infectious Disease Diagnostics, by Purpose of Test, Through 2028
Table 89: Global Market for Clinical NGS-Based Microbiology and Infectious Disease Diagnostics, by Test Complexity, Through 2028
Table 90: NGS in Infectious Disease Diagnostics
Table 91: Global Market for Clinical NGS in Transplantations, by Organ, Through 2028
Table 92: Transplant Procedures Performed in the U.S., by Organ, 2018-2020
Table 93: Global Market for Clinical NGS in Transplantation, by Purpose of Test, Through 2028
Table 94: Global Market for Clinical NGS in Transplantation, by Test Complexity, Through 2028
Table 95: Global Market for Clinical NGS, by Region, Through 2028
Table 96: Global Market for Clinical NGS in Oncology, by Region, Through 2028
Table 97: Global Market for NGS in Reproductive Health, by Region, Through 2028
Table 98: Global Market for Clinical NGS in Transplantation, by Region, Through 2028
Table 99: Patent Activity on Circulating Tumor Cells (CTCs), by Country/Region, January 2010-June 2020
Table 100: List of Select Patents Filed for Circulating Tumor Cells, 2020-2023
Table 101: Patent Activity on Exosomes, by Country/Region, January 2010-December 2020
Table 102: List of Select Patents Filed for Exosomes, 2020-2023
Table 103: Patent Activity on Cell-Free DNA, by Country/Region, January 2010-December 2020
Table 104: List of Select Patents Filed for Cell-Free DNA, 2020-2023
Table 105: Patent Activity on Cancer Biomarkers, by Country/Region, January 2010-December 2020
Table 106: List of Select Patents Filed for Cancer Biomarkers, 2020-2023
Table 107: Status of NGS-Based Liquid Biopsy Patent Disputes, 2023
Table 108: Global Market Ranking, 2022
Table 109: Agilent Technologies Inc.: Company Snapshot
Table 110: Agilent Technologies Inc.: Financial Performance, 2021 and 2022
Table 111: BGI Genomics Co. Ltd: Company Snapshot
Table 112: Illumina Inc.: Company Snapshot
Table 113: Illumina Inc.: Financial Performance, 2021 and 2022
Table 114: Qiagen: Company Snapshot
Table 115: Qiagen NV: Financial Performance, 2021 and 2022
Table 116: Thermo Fisher Scientific Inc.: Company Snapshot
Table 117: Thermo Fisher Scientific Inc.: Financial Performance, 2021 and 2022
Table 118: AccuraGen Holdings: Company Snapshot
Table 119: Adaptive Biotechnologies.: Company Snapshot
Table 120: Alcen: Company Snapshot
Table 121: Ambry Genetics: Company Snapshot
Table 122: Amoy Diagnostics Co. Ltd.: Company Snapshot
Table 123: Angle Plc: Company Snapshot
Table 124: Apostle Sciences: Company Snapshot
Table 125: Arcedi Biotech ApS: Company Snapshot
Table 126: Armonica Technologies Inc.: Company Snapshot
Table 127: Arup Laboratories: Company Snapshot
Table 128: Asuragen Inc.: Company Snapshot
Table 129: Baylor Genetics: Company Snapshot
Table 130: BD: Company Snapshot
Table 131: Berry Genomics Beijing: Company Snapshot
Table 132: Biocaptiva Ltd.: Company Snapshot
Table 133: Biocept Inc.: Company Snapshot
Table 134: Biodesix: Company Snapshot
Table 135: BioFluidica: Company Snapshot
Table 136: Biolidics Ltd.: Company Snapshot
Table 137: Biological Dynamics: Company Snapshot
Table 138: biomodal: Company Snapshot
Table 139: Bionano Genomics: Company Snapshot
Table 140: Bio-Rad Laboratories Inc: Company Snapshot
Table 141: Bio-Techne: Company Snapshot
Table 142: C2i Genomics: Company Snapshot
Table 143: Capio Biosciences: Company Snapshot
Table 144: CareDx Inc.: Company Snapshot
Table 145: Caris Life Sciences: Company Snapshot
Table 146: CeGaT GmbH: Company Snapshot
Table 147: Cell Microsystems: Company Snapshot
Table 148: Centrillion Technologies: Company Snapshot
Table 149: Claret Bioscience: Company Snapshot
Table 150: ClearNote Health: Company Snapshot
Table 151: ClearNote Health: Company Snapshot
Table 152: Cyclomics: Company Snapshot
Table 153: Cygnus Biosciences Co. Ltd: Company Snapshot
Table 154: Danaher: Company Snapshot
Table 155: Dante Labs: Company Snapshot
Table 156: Datar Cancer Genetics: Company Snapshot
Table 157: Delfi Diagnostics: Company Snapshot
Table 158: DiaCarta: Company Snapshot
Table 159: Diagnologix LLC: Company Snapshot
Table 160: Diagnomics Inc.: Company Snapshot
Table 161: Diamir Bio: Company Snapshot
Table 162: DNAlytics: Company Snapshot
Table 163: DNAnexus, Inc.: Company Snapshot
Table 164: EarlyDiagnostics: Company Snapshot
Table 165: Eone Diagnomics Genome Center Co. Ltd: Company Snapshot
Table 166: Epic Sciences: Company Snapshot
Table 167: Epigenomics AG: Company Snapshot
Table 168: Eurofins Genomics: Company Snapshot
Table 169: Everly Health Inc.: Company Snapshot
Table 170: Exact Sciences Corp.: Company Snapshot
Table 171: Exopert: Company Snapshot
Table 172: Exosomics Inc.: Company Snapshot
Table 173: EZlife Bio: Company Snapshot
Table 174: Fabric Genomics Inc.: Company Snapshot
Table 175: F. Hoffmann-La Roche Ltd: Company Snapshot
Table 176: Fluxion Biosciences Inc.: Company Snapshot
Table 177: Freenome Holdings Inc.: Company Snapshot
Table 178: Fulgent Genetics: Company Snapshot
Table 179: Full Genomes Corp.: Company Snapshot
Table 180: Gene by Gene Inc.: Company Snapshot
Table 181: GeneDx LLC: Company Snapshot
Table 182: Geneseq Biosciences: Company Snapshot
Table 183: GenomOncology LLC: Company Snapshot
Table 184: GenoSaber: Company Snapshot
Table 185: Grail Inc.: Company Snapshot
Table 186: Guardant Health: Company Snapshot
Table 187: Helio Genomics: Company Snapshot
Table 188: Helix Inc.: Company Snapshot
Table 189: HTG Molecular Diagnostics Inc.: Company Snapshot
Table 190: Imagia Canexia Health: Company Snapshot
Table 191: IncellDx: Company Snapshot
Table 192: Inex Innovate Private Ltd.: Company Snapshot
Table 193: INOVIQ: Company Snapshot
Table 194: Interpace Biosciences Inc.: Company Snapshot
Table 195: Invitae Corp.: Company Snapshot
Table 196: Invivoscribe Inc.: Company Snapshot
Table 197: Jabrehoo Med Tech Co. Ltd.: Company Snapshot
Table 198: JBS Science: Company Snapshot
Table 199: Jumpcode Genomics Inc.: Company Snapshot
Table 200: Koninklijke Philips N.V.: Company Snapshot
Table 201: Labgenomics: Company Snapshot
Table 202: Laboratory Corporation of America Holdings: Company Snapshot
Table 203: Liquid Biopsy Labs: Company Snapshot
Table 204: Lucence Health Inc.: Company Snapshot
Table 205: LungLife AI: Company Snapshot
Table 206: Macrogen Inc.: Company Snapshot
Table 207: MapmyGenome: Company Snapshot
Table 208: mdxhealth: Company Snapshot
Table 209: MedGenome: Company Snapshot
Table 210: Medicover Genetics: Company Snapshot
Table 211: Menarini Silicon Biosystems: Company Snapshot
Table 212: Merck KGaA: Company Snapshot
Table 213: MiCareo Inc.: Company Snapshot
Table 214: Micronoma INC.: Company Snapshot
Table 215: miR Scientific: Company Snapshot
Table 216: MutantDx: Company Snapshot
Table 217: Myriad Genetics, Inc.: Company Snapshot
Table 218: NanoString Technologies Inc.: Company Snapshot
Table 219: Natera Inc.: Company Snapshot
Table 220: Nebula Genomics Inc.: Company Snapshot
Table 221: NeoGenomics Laboratories: Company Snapshot
Table 222: New England Biolabs: Company Snapshot
Table 223: New Horizon Health: Company Snapshot
Table 224: NOVIGENIX SA: Company Snapshot
Table 225: Novogene Co Ltd: Company Snapshot
Table 226: Nrichdx: Company Snapshot
Table 227: NuProbe: Company Snapshot
Table 228: NX Prenatal Inc.: Company Snapshot
Table 229: Oncimmune Holdings PLC: Company Snapshot
Table 230: OncoCyte Corp.: Company Snapshot
Table 231: OncoDNA: Company Snapshot
Table 232: OPKO Health Inc.: Company Snapshot
Table 233: Orchid: Company Snapshot
Table 234: Oxford Nanopore Technologies plc: Company Snapshot
Table 235: PacBio: Company Snapshot
Table 236: Pangaea Oncology: Company Snapshot
Table 237: Personal Genome Diagnostics Inc: Company Snapshot
Table 238: Personalis Inc.: Company Snapshot
Table 239: Phase Scientific: Company Snapshot
Table 240: PierianDx: Company Snapshot
Table 241: Predicine: Company Snapshot
Table 242: Prenetics Ltd.: Company Snapshot
Table 243: QCDx: Company Snapshot
Table 244: Quantapore: Company Snapshot
Table 245: Quantgene Inc.: Company Snapshot
Table 246: QuantuMDx Group Ltd.: Company Snapshot
Table 247: Quest Diagnostics: Company Snapshot
Table 248: Rarecells Inc.: Company Snapshot
Table 249: Ravgen.: Company Snapshot
Table 250: Real Time Genomics.: Company Snapshot
Table 251: Resolution Bioscience Inc.: Company Snapshot
Table 252: SAGA Diagnostics.: Company Snapshot
Table 253: Sano Genetics: Company Snapshot
Table 254: ScreenCell: Company Snapshot
Table 255: SeekIn Inc.: Company Snapshot
Table 256: Sequencing.Com: Company Snapshot
Table 257: Seven Bridges Genomics: Company Snapshot
Table 258: Single Technologies AB: Company Snapshot
Table 259: SmartCatch: Company Snapshot
Table 260: StageZero Life Sciences. Ltd.: Company Snapshot
Table 261: Strand: Company Snapshot
Table 262: Strata Oncology Inc.: Company Snapshot
Table 263: Syapse Inc.: Company Snapshot
Table 264: Sysmex Inostics Inc.: Company Snapshot
Table 265: Takara Bio Inc: Company Snapshot
Table 266: Telexos GmbH: Company Snapshot
Table 267: TwinStrand Biosciences Inc.: Company Snapshot
Table 268: Twist Bioscience: Company Snapshot
Table 269: Unchained Labs: Company Snapshot
Table 270: Universal DX: Company Snapshot
Table 271: Vela Diagnostics: Company Snapshot
Table 272: Veracyte Inc.: Company Snapshot
Table 273: VolitionRx Ltd.: Company Snapshot
Table 274: Vortex Biosciences: Company Snapshot
Table 275: Yourgene Health: Company Snapshot
Table 276: Yikon Genomics: Company Snapshot
List of Figures
Summary Figure: Global Market for Clinical Applications of Next-Generation Sequencing, by Disease Class, 2020-2028
Figure 1: Timeline of the Advancement of Sequencing Technology
Figure 2: How A Strong ESG Proposition Benefits Businesses
Figure 3: The Ten Business Sustainability Trends Identified for 2022
Figure 4: ESG-Adoption Level Across All the Industries, 2021 and 2022
Figure 5: Key Sustainable Practices Being Implemented in Research Laboratories
Figure 6: Agilent Technologies Inc.: Financial Performance, 2021 and 2022
Figure 7: Agilent Technologies Inc.: Revenue Share, by Business Unit, 2022
Figure 8: Agilent Technologies Inc.: Revenue Share, by Region, 2022
Figure 9: Illumina Inc: Financial Performance, 2021 and 2022
Figure 10: Illumina Inc.: Revenue Share, by Business Unit, 2022
Figure 11: Illumina Inc.: Revenue Share, by Country/Region, 2022
Figure 12: Qiagen NV: Financial Performance, 2021 and 2022
Figure 13: Qiagen NV: Revenue Share, by Business Unit, 2022
Figure 14: Qiagen NV: Revenue Share, by Region, 2022
Figure 15: Thermo Fisher Scientific Inc.: Financial Performance, 2021 and 2022
Figure 16: Thermo Fisher Scientific Inc.: Revenue Share, by Business Unit, 2022
Figure 17: Thermo Fisher Scientific Inc.: Revenue Share, by Region, 2022